
First-line application of apatinib combined with S-1 based on peripheral circulating tumor cell screening to treat advanced gastric adenocarcinoma: a case report
Introduction
Gastric cancer (GC) is the third most commonly diagnosed malignancy, with an annual incidence of 30.00/100,000, and the third most common cause of cancer-related deaths in China, with a mortality of 21.48/100,000, in 2014 (1,2). In China, approximately 90% of GC cases are discovered at an advanced stage. The 5-year survival rate of GC patients is still less than 40%. Thus, compared to that in Japan or other countries, there is a large gap between the rates of diagnosis and survival (3,4). The advent of targeted therapy may improve survival in patients with advanced GC (AGC).
Apatinib is a highly selective oral tyrosine kinase inhibitor (TKI) for vascular endothelial growth factor receptor-2 (VEGFR-2). As a third-line therapeutic regimen, apatinib has been recommended for stage IA disease (5). Circulating tumor cells (CTCs), first identified by Dr. Ashworth in 1869, are released into blood circulation from primary or metastatic tumors, and these cells have specific antigens and genetic characteristics (6,7). Patients with a CTC-high status before and during chemotherapy usually have a poor prognosis (8). Until now, there has been no report in the literature of the use of apatinib as a first-line treatment for patients with CTC-positive GC. We present a case of an elderly Chinese man with peripheral circulating tumor cell (pCTC)-positive disease whose GC-derived liver metastases demonstrated partial response to persistent apatinib treatment. Surprisingly, the patient’s pCTCs completely disappeared when apatinib was combined with S-1.
Case presentation
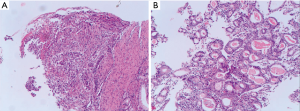
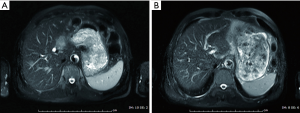
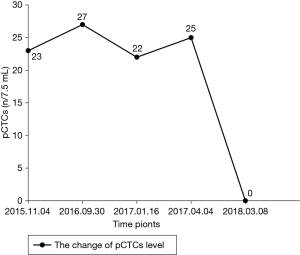
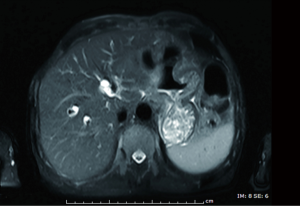
A 71-year-old man presented with epigastric distension and discomfort for 2 months, which had become aggravated for 10 days with hematemesis and black stool. The patient had a history of hypertension for 2 years. His maximal blood pressure (BP) was 180/120 mmHg. His BP was well controlled with nifedipine sustained release tablets and valsartan. On November 4, 2015, the patient was diagnosed with moderately differentiated advanced gastric adenocarcinoma located in the lesser curvature of the body with multiple liver and abdominal lymph node metastases based on imaging examination, gastroscopic biopsy and laparoscopy (Figures 1,2A). The patient had lost the opportunity for surgical intervention. At this point, his pCTC count was 23. The patient’s relatives refused intravenous chemotherapy because of his poor health and wanted him to try targeted drugs. On November 17, the patient was discharged and began to take apatinib alone (850 mg po qd) after signing an informed consent form refusing chemotherapy. On September 30, 2016, the patient developed intermittent headache, dizziness and other symptoms, with a BP of 163/90 mmHg, foamy urine and a pCTC count of 27. Abdominal magnetic resonance imaging (MRI) showed that the liver metastases were smaller than before. The dose of apatinib was decreased to 750 mg po qd. On January 16, 2017, the patient began to feel better after apatinib dose was decreased. Abdominal MRI showed that liver metastases were even smaller than the last evaluation. The dose of apatinib was increased to 850 mg, and the pCTC count was 22. On April 4, 2017, the patient’s liver metastases disappeared (Figure 2B). Emission computed tomography (ECT) showed no obvious metastasis, and the pCTC count was 25. The patient began to take S-1 at 40 mg po bid. Until March 13, 2018, the patient’s CTC count was 0. During this period, reexamination of CTCs showed a decreasing trend (Figure 3) . After 1 month, the patient experienced difficulty in eating. On May 8, 2018, MRI showed that the size and shape of the liver were normal, and there was no obvious abnormal signal in the liver parenchyma. However, the wall of the gastric body and fundus was significantly thicker than before, indicative of tumor progression (Figure 4). The patient’s family members refused long-term nutritional support treatment. On June 2, 2018, the patient died of cachexia. His overall survival (OS) was 34 months.
Discussion
Metastasis and recurrence of GC significantly hamper prognosis. Due to the characteristic anatomical feature and venous supply, the liver is the most frequent site of GC metastasis. Approximately 4–14% of patients with AGC have multiple liver metastases, which are often accompanied by local invasion, peritoneal lesions, and distant lymph node involvement. The median OS (mOS) is shorter than 6 months (9-12). CTCs from the primary or metastatic tumor spontaneously enter peripheral circulation during diagnostic and therapeutic interventions. These cells circulate, disseminate and eventually leave the blood circulation to form metastatic lesions in distant tissues or organs (13). Many studies have confirmed that CTCs are strongly associated with palindromia, metastasis and prognosis of GC (14). At present, the Food and Drug Administration (FDA) has approved CTC testing for breast cancer, colon cancer and prostate cancer. Many studies have shown that for breast cancer and prostate cancer, 5 CTCs/7.5 mL peripheral blood can be used as an indicator of poor prognosis (15,16). Onstenk et al. reported that the gene expression profile of CTCs in advanced colon cancer was more similar to the profile of liver metastases (74%) than that of primary tumors (57%) (17). Pizon et al. found that VEGFR-2 is expressed on CTCs in 84% of patients with breast cancer (18). Based on these data, we can boldly speculate that patients with liver metastasis from AGC also have similar characteristics. In recent years, a rapid development of negative enrichment methods has been observed, and these methods have been widely used in the clinic. In these methods, target cells are obtained by gradually removing blood cells (using red blood cell lysis, specific antibodies and the leukocyte surface antigen CD45). This technique is easy to perform, can be widely used, can yield more stable target cells, and can effectively enrich for CTCs from various solid tumors (19). We enriched for pCTCs by density gradient centrifugation and immunomagnetic negative enrichment and then used fluorescence in situ hybridization and immunofluorescence staining to detect and identify the CTCs. We used a threshold of more than 5 CTCs/7.5 mL blood as positive. Finally, we observed and counted the cells under a fluorescence microscope. Apatinib is a TKI that can block CTCs from forming micrometastases.
Traditional intravenous chemotherapy leads to malignant cell death by interfering with intracellular nucleic acid metabolism. With more than 60 years of research, development and improvement, traditional chemotherapy shows some therapeutic effect in clinical settings. A recent retrospective multicenter cohort study revealed that cisplatin is effective in promoting transient tumor shrinkage and stability, but it cannot prolong the survival of elderly patients with AGC (20). Additionally, further increase in efficacy is restricted by resistance and toxicity. Thus, cytotoxic therapies for GC have reached a bottleneck. The scope of molecular targeting agents, which have become a palliative treatment option for AGC, is rapidly expanding. The results from ToGA (Trastuzumab for Gastric Cancer) confirmed the curative effect of trastuzumab therapy for HER-2-positive GC, and the door for targeted treatment of GC was formally opened (21). To date, various studies have shown that the mOS of patients with HER-2-positive advanced gastric/gastroesophageal junction adenocarcinoma can be as high as 16.4 months in response to trastuzumab (22). However, in other studies, molecular targeting agents such as bevacizumab and cetuximab failed in the treatment of GC (23,24). In 2015, GC was second only to lung cancer in our country in terms of morbidity and mortality, thereby ranking second among all malignant tumors. Hundreds of thousands of GC patients are waiting for high-performance antineoplastic protocols in China (15). Therefore, we need to carefully consider whether it is desirable to exchange high treatment efficiency for low quality of life. In this case, to improve patient compliance and quality of life, we regularly monitor pCTCs, biochemical/hematological parameters, vital signs, clinical features and radiological images under minimally or no invasive procedures. Based on the findings, we often change oral apatinib or S-1.
Apatinib, a targeted therapeutic drug independently developed in China, is a specific TKI targeting VEGFR-2 and disrupts the binding of VEGFR-2 to VEGF, thereby inhibiting neoplastic processes (12). Multicenter randomized, double-blind, controlled phase II/III clinical trials in GC have shown that apatinib could significantly prolong patient progression-free survival (PFS) and OS (21,25). Apatinib was approved in China by the China General Administration of Food and Drug Administration (CDFA) in 2014 and recommended by the Chinese Society of Clinical Oncology (CSCO) as a third-line therapy for metastatic GC. Nevertheless, strategies to fully utilize apatinib remain controversial. In a phase III clinical trial, 267 patients with AGC who developed metastasis were divided into an apatinib administration group (AG: n=176) and a placebo group (PG: n=91), and OS and PFS were the primary and secondary study endpoints, respectively [mOS: AG/PG =4.7/1.8 mon, P=0.165; median PFS (mPFS): AG/PG=6.5/2.6, P<0.01] (21). The results indicated that patients could benefit from apatinib alone. Makiyama et al. have shown that combined therapy is more impactful for GC than chemotherapy alone. Nevertheless, combined therapy increases the rate of complications at all levels (20). The clinical curative effect in elderly patients is considerable. Compared with the above study results, the patient's outcome in this report was satisfactory. It is possible that apatinib not only resolves multiple liver metastases originating from GC but also efficiently prevents their reemergence when combined with S-1 without severe complications in China.
In the present case, the patient’s liver metastases originating from GC showed a partial response to apatinib with relatively high compliance, good tolerance and no severe complications. Although CTCs, indicative of metastasis, were positive throughout treatment, metastasis did not reemerge before the patient started taking S-1. The antitumor effect of apatinib has been well demonstrated. Of course, we need to examine more cases to verify this conclusion. Our patient began to take S-1 alone after developing grade 2 hand-foot syndrome. When the condition was significantly relieved, the patient continued to take oral apatinib combined with S-1 for 13 months. Surprisingly, his pCTC status became negative until death. To date, there is no evidence supporting the use of pCTCs as an indication for combined apatinib and S-1 therapy. Whether pCTC positivity can be used as an indication for the first-line application of this combined regiment in the clinic is worth exploring. Unfortunately, because of personal reasons, the patient refused to undergo genetic screening. Furthermore, the pathological section used for diagnosis was not from our hospital. Therefore, the expression level of VEGFR-2 in the patient remained unclear.
The presence of CTCs usually indicates poor prognosis in response to chemotherapy. This is the first report showing that CTCs can be used as an indication for combined therapy with apatinib and S-1. We believe that the quality of life and survival duration of patients with advanced cancer should take precedence over the removal of multiple metastatic foci. The combined application of molecular targeted agents guided by genetic screening technologies and serological indexes may become the main strategies for future cancer treatment. Thus, the relationship between CTCs and liver metastasis in GC is worth exploring.
Acknowledgements
Funding: This study was supported by the Huimin Plan of Ministry of Science and Technology & Ministry of Finance, China (grant No. 2012GS620101). the Major Projects of Science and Technology in Gansu Province of China (grant No. 2011GS04390), the Natural Science Foundation of in Gansu Province of China (grant No. 1506RJZA309), and the Postdoctoral Research Foundation of China (grant No. 2015M572710).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the patient’s wife in the study.
References
- Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:1-12. [Crossref] [PubMed]
- Venerito M, Link A, Rokkas T, et al. Gastric cancer - clinical and epidemiological aspects. Helicobacter 2016;21 Suppl 1:39-44. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Katai H, Ishikawa T, Akazawa K, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer 2018;21:144-54. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Ashworth TR. A Case of Cancer in Which Cells Similar to Those in the Tumors Were Seen in the Blood after Death. Aust Med J 1869;14:146-9.
- Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumor cells. Science 2013;341:1186-8. [Crossref] [PubMed]
- Zou K, Yang S, Zheng L, et al. Prognostic Role of the Circulating Tumor Cells Detected by Cytological Methods in Gastric Cancer: A Meta-Analysis. Biomed Res Int 2016;2016:2765464. [Crossref] [PubMed]
- Sakamoto Y, Ohyama S, Yamamoto J, et al. Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery 2003;133:507-11. [Crossref] [PubMed]
- Okano K, Maeba T, Ishimura K, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg 2002;235:86-91. [Crossref] [PubMed]
- Sakamoto Y, Sano T, Shimada K, et al. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol 2007;95:534-9. [Crossref] [PubMed]
- Marrelli D, Roviello F, De Stefano A, et al. Risk factors for liver metastases after curative surgical procedures for gastric cancer: a prospective study of 208 patients treated with surgical resection. J Am Coll Surg 2004;198:51-8. [Crossref] [PubMed]
- Zhang ZY, Ge HY. Micrometastasis in gastric cancer. Cancer Lett 2013;336:34-45. [Crossref] [PubMed]
- Uenosono Y, Arigami T, Kozono T, et al. Clinical significance of circulating tumor cells in peripheral blood from patients with gastric cancer. Cancer 2013;119:3984-91. [Crossref] [PubMed]
- Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23:1420-30. [Crossref] [PubMed]
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9. [Crossref] [PubMed]
- Onstenk W, Sieuwerts AM, Mostert B, et al. Molecular characteristics of circulating tumor cells resemble the liver metastasis more closely than the primary tumor in metastatic colorectal cancer. Oncotarget 2016;7:59058-69. [Crossref] [PubMed]
- Pizon M, Zimon DS, Pachmann U, et al. Insulin-like growth factor receptor I (IGF-IR) and vascular endothelial growth factor receptor 2 (VEGFR-2) are expressed on the circulating epithelial tumor cells of breast cancer patients. PLoS One 2013;8:e56836. [Crossref] [PubMed]
- Li Y, Zhang X, Ge S, et al. Clinical significance of phenotyping and karyotyping of circulating tumor cells in patients with advanced gastric cancer. Oncotarget 2014;5:6594-602. [Crossref] [PubMed]
- Makiyama A, Kunieda K, Noguchi M, et al. First-line chemotherapy with S-1 alone or S-1 plus cisplatin for elderly patients with advanced gastric cancer: a multicenter propensity score matched study. Gastric Cancer 2018;21:792-801. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Palacio S, Loaiza-Bonilla A, Kittaneh M, et al. Successful use of Trastuzumab with anthracycline-based chemotherapy followed by trastuzumab maintenance in patients with advanced HER2-positive gastric cancer. Anticancer Res 2014;34:301-6. [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Hu B, El Hajj N, Sittler S, et al. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol 2012;3:251-61. [PubMed]
- Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219-25. [Crossref] [PubMed]


