
Population screening and diagnostic strategies in screening family members of Wilson’s disease patients
Introduction
Wilson’s disease (WD), also named hepatolenticular degeneration, is an autosomal-recessive disorder in which abnormal copper metabolism leads to copper excretion disorder and deposition in the liver, nervous system and other parts, thus causing dysfunction of the corresponding organs. The Kayser-Fleischer (KF) rings reflect deposition of copper in the cornea.
WD was first described in detail in 1912 as a progressive, hereditary, and invariably fatal disease (1). The clinical manifestation of WD is highly variable, ranging from patients who are asymptomatic between birth to the onset to patients gradually appear the symptoms of organ damage with the increase of copper deposition in the body. Liver problems caused by WD are mainly manifested as acute and chronic hepatitis, liver cirrhosis, fulminant hepatitis (2). Neurological symptoms are mainly neuropsychiatric symptoms and extrapyramidal symptoms (3,4). Other accompanying symptoms include hemolytic anemia, haematuria albuminuria, a mental and psychological disorder.
The ATP7B gene is considered as the key pathogenic gene for WD, which encodes a transmembrane protein ATPase (ATP7B). ATP7B is a P-type ATPase, which is involved in copper combining into ceruloplasmin and accelerate copper excretion from the biliary tract. Mutations in the ATP7B gene leads to absent or markedly decrease of ATP7B levels, which results in excessive deposition of copper in the liver, brain and other organs (5). Up to now, more than 600 mutations in the ATP7B gene have been reported (6).
Most studies reported a worldwide prevalence of WD to be 1 in 30,000 (30 per million) and a carrier rate of about 1 in 90. But, these dates were determined before the ATP7B gene was identified. WD is not always easy to been recognized due to the variety of clinical manifestation. Thus, the prevalence rate of WD is likely to be underestimated. In a gene study in the United Kingdom, 6,384 individuals were selected and their gene was sequenced (the full-length ATP7B gene in 1,008 individuals and exons 8, 14, 18 in 5,376 individuals, respectively). The result indicated the most conservative frequency of individuals with WD is 1:7,026 and thus higher than the reported prevalence of 1:30,000 (7). Another cause for underestimating the prevalence of WD in a population is that risk for offspring (8) and previous generation suffering from WD is underestimated. Some studies have found that WD exists not only in siblings but also in two or three consecutive generations (9-11). In addition, regional variations exist, there is a higher prevalence in people who are isolated or have high rates of intermarriage, for example, Costa Rica, Sardinia, Canary islands and Crete (12-15).
WD has high mortality and disability rate (16), however, it is treatable. Irreversible tissue injury may not occur if WD was diagnosed and treated before the development of clinical symptoms (17). Individuals identified as patients by familial screening should be treated. Therefore, it is necessary to screen WD in the population, especially in the family members of the proband with WD.
Family screening
Screening range for the family member
As an autosomal recessive genetic disease, WD is highly recommended to perform familial screening, American Association for the Study of Liver Diseases (AASLD) (18) and European Association for the Study of the Liver (EASL) (19) recommend screening first degree relatives of a proband. First-degree relatives not only include the siblings of a proband, but also the offspring and parents of a proband. In practice, this instruction has often been explained as focused on siblings while underestimated the risk to parents and offspring to have WD. It is frequently considered that WD occurs in siblings (25%), but it also occurs in the previous generation (0.5%) and the offspring (0.5%), although rarely reported (20). Even though this risk is low, screening parents and the children of a proband is justified given the potential devastating process of WD. Parents are often considered heterozygous. Although most of WD patients were younger than 40 years, several researchers reported old patients diagnosed with WD in their early 80. It is reported that (21) a 43-year-old asymptomatic father screened and confirmed WD after his daughter was diagnosed with a typical WD. In our previous study, a 41-year-old mother of a proband was also diagnosed as a presymptomatic patient with WD through whole ATP7B gene sequencing (22). Considering incidence equaling to the previous generation, screening for the next generation is also necessary. A study in two Sardinian families found two atypical patients with WD in offsprings by genetic testing. Other similar findings have been reported (9,11).
WD show different clinical symptoms including hepatic, neurological, psychiatric disorder, and asymptomatic (20,23), and the phenotype often differ among patients with the same genotype, even within a single family (24). Considering the underestimation of WD incidence, the probability of late-onset and asymptomatic, and differ phenotype of the same genotype, it seems vital to screen the previous and next generation of the proband. In addition, it needs to be emphasized that the patients diagnosed with WD in childhood or adolescence need to be taught, once they have children, the children should be screening for WD.
The probability of nephews and nieces being affected is 1 in 600, and the probability of cousins being affected is 1 in 800 (25), which is significantly higher than that of the general population. In a family with affected members over two generations, an uncle and two cousins were diagnosed as WD after the proband was diagnosed as WD (26). All members of this family came from the same village, but there was no known relationship by blood in this family. These studies indicate not only first degree relative of probands must be screened for WD, but distant relatives maybe also are considered, especially in certain areas, such as some villages and small islands.
Screening method
First-degree relatives of a proband with WD should be evaluated in these aspects including the history of liver disease and neurological symptoms; physical examination; and liver function tests including aminotransferases, bilirubin, albumin; ceruloplasmin, serum copper, basal 24-h urinary copper, KF rings in eyes. If the results of these tests are insufficient for the diagnosis of WD for some people, hepatic copper concentrations should be measured. Among these, serum ceruloplasmin, 24-h urinary copper excretion, hepatic copper content, and KF ring are important to diagnose WD. If available, mutation analysis or haplotype studies based on findings in a proband should be used as preliminary screening (Figure 1) (18). Genetic analysis is the only test for diagnosing presymptomatic patients and carriers. Therefore, a correct understanding of the meaning and limitations of these diagnostic methods is essential.
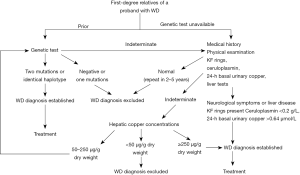
Serum ceruloplasmin
Ceruloplasmin is not encoded by the WD gene (ATP7B) but by the ceruloplasmin gene located on human chromosome 3q24-q25. The newly synthesized ceruloplasmin obtained 6 copper ions transported by ATP7B and became mature ceruloplasmin. If the ATP7B gene is mutated, the precursor of ceruloplasmin will degrade rapidly due to insufficient copper ions, resulting in the reduction of ceruloplasmin. The normal concentration of ceruloplasmin is usually 0.2 to 0.4 g/L. At birth to the age of 6 months, serum ceruloplasmin level is lower than which in the adult. Ceruloplasmin is unstable in the body, it is reduced in cases of Menkes’ disease, nephritic syndrome, protein-losing enteropathy, and 20% of heterozygous carriers (18,27,28). Ceruloplasmin may increase to normal range in WD patients suffering pregnant, taking oral contraceptives, infections, and hepatitis. Some WD patients have normal levels of ceruloplasmin (29,30). Taking a low ceruloplasmin level as a screening test for WD, it had only a positive predictive value of 6% (31). Ceruloplasmin is not a specific indicator for the diagnosis of WD, but the level of serum ceruloplasmin less than 0.1g/L is strong evidence for the diagnosis of WD (32).
Urine copper excretion
In newly diagnosed patients, the 24-h urine copper amount reflects the level of non-ceruloplasmin bound copper in the blood (19). In order to avoid affecting the results of 24 h of urine copper excretion, these aspects need to be noted: complete urine collection, adequate water intake, prevention of contamination and accurate measurement of urine volume. The specificity and sensitivity of 24-h urine copper in the diagnosis of WD is not very high. Assessing 24 h urinary copper levels may also result in misdiagnosis as 24-h urinary copper levels increase in other liver diseases and slightly elevate in carriers (32,33). The normal range is 1.6 µmol/24 h for adults and 0.64 µmol/24 h for children. Some studies suggested reducing the urine copper excretion threshold (without penicillamine stimulation) to 0.64 µmol/24 h improved test sensitivity and eliminated the necessity for the penicillamine challenge test (34,35).
The penicillamine stimulation test refers to 24 h of urine copper when taking penicillamine. It has been clarified in children (36), in which 500 mg of D-penicillamine was taken orally at first and again 12 h later, urine collected 24 h later, body weight regardlessly. This trial was reconfirmed value of diagnosis WD in pediatric patients with active liver disease, but could not exclude the diagnosis of asymptomatic siblings (37). The penicillamine stimulation test is not suitable for diagnosis of adult patients with WD (38,39).
Hepatic copper concentration
The hepatic copper content is generally considered as the gold standard for WD, with the threshold value of ≥250 µg/g dry weight. In 2005, Ferenci et al. proposed that the optimal threshold should be ≥75 µg/g dry weight based on their results. With the threshold reducing from 250 to 75 µg/g, the sensitivity increased from 83.3% to 96.5%, and the specificity was still acceptable (95.4% vs. 98.6%) (40). In the later stage of WD, because of the uneven distribution of copper in the liver, the estimated value of a single biopsy specimen may be misleading. However, when the specimen size is sufficient (tissue core length is at least 1 cm), the accuracy of measurement will be improved (35). Hepatic copper content significantly increases (more than 250 µg/g dry weight) in established cholestatic disorders and idiopathic copper toxicosis syndromes (32), but decreases to normal range in patients with regenerative nodules. The hepatic copper content in heterozygotes is often higher than normal but does not weight over 250 µg/g dry weight. In addition, liver biopsy is an invasive procedure, and safety and patient acceptance are issued to be considered.
KF ring
The KF ring is a golden-brown pigment band caused by copper deposition in the elastic layer behind the corneal limbus, which reflects the accumulation of copper outside the liver. It is more common in WD patients with neurological symptoms (38,41). It is rarely found in children with liver symptoms (29). KF Ring is not a specific index, also may appear in patients with primary biliary liver cirrhosis, chronic activity hepatitis, childhood progressive inside liver cholestasis, alcoholic liver cirrhosis (42,43). Deep jaundice may affect the judgment of KF Ring. Nagral et al. (44) reported that most patients with acute jaundice with total bilirubin >20 mg/dL could be found the KF like Ring, which disappeared after jaundice subsided.
Genetic testing
If available, genetic analysis may be used as the primary screening method in WD family screening. WD gene analysis methods mainly include direct sequencing and haplotyping (19). At present, mutation analysis is widely available and contributes vastly to WD diagnosis (18). Individuals carrying two pathogenic mutations are considered to contain the disease according to the characteristics of autosomal recessive inheritance diseases. Direct sequencing, which identifies the type of mutation, is a standard way of diagnosing WD. Although the test of detecting the ATP7B gene is costly, it will decline if a proband mutation is identified, siblings needing only be analyzed for the same mutation (18). Siblings with only one mutation or none at all were excluded from WD. However, the screening method of the previous generation is different from those for the sibling. The modality that only analyzing the two mutations of a proband is not enough to diagnose WD in these parents. Thus, the complete ATP7B genes of suspected atypical and presymptomatic parents should be sequenced. Similarly, it is also not enough for the next generation to be only screened known mutations of a proband. Haplotyping is suitable in screening the relatives of a proband with WD when mutations in proband are not detected. This analysis requires the identification of proband who have been diagnosed with WD in the family (18). The inheritance of the “disease-associated” haplotypes allows determining whether they are indeed patients, heterozygous or unaffected (27). If haplotypes are used for low probability gene recombination, false positive results may occur (45). Gene diagnosis of WD will be more efficient and comprehensive by developing new sequencing technology. In view of the fact that patients diagnosed by family screening are usually presymptomatic, gene analysis is recommended for their diagnosis (19). Meanwhile, gene analysis differentiates heterozygous carriers of WD (WDHzc) from patients with WD and it also avoids continuous testing if the results were not enough to diagnose or exclude WD. We used to screen a family for WD, and we found two presymptomatic patients by a genetic test, which resulted in a proper initiation of therapy (23). Similar reports have been widely reported (20,46).
WD score (Leipzig score)
WD diagnosis is a comprehensive diagnosis rather than a single evidential diagnosis. A single symptom, sign or laboratory index is not sufficient to determine or exclude WD. Negative gene test also cannot exclude WD. In order to get high diagnostic accuracy, WD scoring system based on available tests was suggested by the Working Party at the 8th International Meeting on WD, Leipzig 2001 The working group presented a WD scoring system based on existing tests at the 8th International Meeting on WD in Leipzig, Germany in 2001 (47). WD is likely to be diagnosed with a score greater than or equal to 4, and it is highly unlikely to be diagnosed, with a score less than or equal to 2. This score was recommended for the diagnosis of WD in the recent EASL clinical guidelines (19).
In the application of the diagnostic integral system, we must pay attention to the following aspects: (I) Individuals without KF rings but with subnormal ceruloplasmin and abnormal liver functions should be confirmed by liver biopsy or mutation analysis. (II) Situations that lead to false positives and false negatives for each indicator should be excluded when using this system. (III) If a suspect patient hasn’t had all the tests including copper liver and genetic tests, even if the score is low, WD cannot be ruled out easily. (IV) Patients with cholestasis may present with elevated hepatic copper (2 points) and a KF ring (2 points), which brings the score up to four points. (V) The carrier incorporates another disease to be like hepatitis B may show elevated levels of ceruloplasmin (1 point), 24-h urinary copper levels (1 point), and liver copper (1 point), 1 mutation (2 points), even KF ring (2 points), with a total score greater than or equal to 4. Patients with a score of 4 or above are more likely to be diagnosed with WD. The above situation requires a typical genotyping. A genetic test may be the most practical method for the diagnosis of WD in children, carrier and presymptomatic patients.
Although family members of WD proband are highly suspected of having the same disease, the diagnosis must be based on sufficient clinical evidence and laboratory data to avoid misdiagnosis leading to unnecessary lifelong treatment and complications. Strong family history can be a diagnostic bias if the member of WD’s family is a heterozygous carrier for WD with other liver or neurological conditions. Carriers for WD tend to have some abnormal biochemical indicators, and they may be misdiagnosed as WD without adequate examination. The carriers do not have WD and do not require treatment (48).
General population screening
WD’s routine population screening is not universal. It is commonly believed that due to low prevalence and lack of insensitive biomarkers, large-scale screening in the general population for WD was considered neither feasible nor cost-efficient. However, WD meets the criteria for neonatal screening: Its actual incidence is not low; it can be treated and the prognosis is good (8). As mentioned above, although ceruloplasmin 24-h urine copper excretion, KF ring, and hepatic copper concentration are all important indicators for the diagnosis of WD, they are all limited and not specific indicators for the diagnosis of WD. None of them are appropriate for screening in populations or newborns. As the most accurate method at present, gene screening is neither economical nor practical for general population screening because of its high cost. Nevertheless, it is a promising strategy in limited populations with the same prevalent mutations. For example, people in certain islands or villages often have the same genetic mutation. Screening for known types of mutations in these populations is an economical and efficient method.
Current neonatal screening uses mass spectrometry to quantitatively detect proteins in newborn dried blood spots to screen for other inherited disorders. With respect to WD, given the limitation of ceruloplasmin, this can be done for ATP7b protein itself. Jung et al. used immunoaffinity liquid chromatography-tandem mass spectrometry (LC-MS/MS) check to quantitatively detect the levels of ATP7b 1056 peptide in dried blood spots (49). The study successfully distinguished patients with WD from the carriers and normal controls. While the results of the research seem promising, it still needs to be determined whether such a method can be used for screening in the general population. At first, the sample size of this study is small, it is necessary to expand the sample size, especially for carriers and WD patients with a wide spectrum of mutations. In addition, the study is only for children or adult. and no neonates were studied. Since the effect of age on ATP7B protein levels (especially in neonates) is unknown, if it is to be used as a neonatal WD screen, as with all new born screening trials, the trial will need to be conducted in the neonates and/or infants.
In the early stages of WD disease, it is difficult to detect patients because symptoms may be completely absent or nonspecific, especially in children and asymptomatic adults. However, for patients with extrapyramidal disorders, liver diseases and elevated liver tests for unknown reasons found in physical examination, the physician should consider the possibility of WD diagnosis and select appropriate diagnostic tests.
Conclusions
First-degree relatives of a proband with WD should be genetically screened. First-degree relatives should include the previous generation, siblings and the next generation. If available, genetic testing should be used as the primary screening family members method. Although the relatives of a proband are more likely to be patient with WD, the diagnosis should be based on sufficient evidence to avoid unnecessary lifelong treatment. So far, there is no reliable test for WD screening in the general population including neonates. However there have been new explorations through the study on ATP7B protein, but further research is needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wilson SAK. Progressive lenticular degeneration-a familial nervous disease associated with cirrhosis of the liver. Brain 1912;34:295-509. [Crossref]
- Beinhardt S, Leiss W, Stättermayer AF, et al. Long-term outcomes of patients with Wilson disease in a large Austrian cohort. Clin Gastroenterol Hepatol 2014;12:683-9. [Crossref] [PubMed]
- Seniów J, Mroziak B, Czlonkowska A, et al. Self-rated emotional functioning of patients with neurological or asymptomatic form of Wilson’s disease. Clin Neuropsychol 2003;17:367-73. [Crossref] [PubMed]
- Zimbrean PC, Schilsky ML. Psychiatric aspects of Wilson disease: A review. Gen Hosp Psychiatry 2014;36:53-62. [Crossref] [PubMed]
- Dong QY, Wu ZY. Advance in the pathogenesis and treatment of Wilson disease. Transl Neurodegener 2012;1:23. [Crossref] [PubMed]
- Aggarwal A, Bhatt M. Update on Wilson disease. Int Rev Neurobiol 2013;110:313-48. [Crossref] [PubMed]
- Coffey AJ, Durkie M, Hague S, et al. A genetic study of Wilson's disease in the United Kingdom. Brain 2013;136:1476-87. [Crossref] [PubMed]
- Roberts EA. Update on the Diagnosis and Management of Wilson Disease. Curr Gastroenterol Rep 2018;20:56. [Crossref] [PubMed]
- Dufernez F, Lachaux A, Chappuis P, et al. Wilson disease in offspring of affected patients: report of four French families. Clin Res Hepatol Gastroenterol 2013;37:240-5. [Crossref] [PubMed]
- Loudianos G, Zappu A, Lepori MB, et al. Wilson’s disease in two consecutive generations: the detection of three mutated alleles in the ATP7B gene in two Sardinian families. Dig Liver Dis 2013;45:342-5. [Crossref] [PubMed]
- Bennett JT, Schwarz KB, Swanson PD, Hahn SH. An exceptional family with three consecutive generations affected by Wilson disease. JIMD Rep 2013;10:1-4. [PubMed]
- Shah AB, Chernov I, Zhang HT, et al. Identification and analysis of mutations in the Wilson disease gene (ATP7B): population frequencies, genotype-phenotype correlation, and functional analyses. Am J Hum Genet 1997;61:317-28. [Crossref] [PubMed]
- Giagheddu A, Demelia L, Puggioni G, et al. Epidemiologic study of hepatolenticular degeneration (Wilson’s disease) in Sardinia (1902-1983). Acta Neurol Scand 1985;72:43-55. [Crossref] [PubMed]
- Loudianos G, Dessi V, Lovicu M, et al. Molecular characterization of wilson disease in the Sardinian population--evidence of a founder effect. Hum Mutat 1999;14:294-303. [Crossref] [PubMed]
- Dedoussis GV, Genschel J, Sialvera TE, et al. Wilson disease: high prevalence in a mountainous area of Crete. Ann Hum Genet 2005;69:268-74. [Crossref] [PubMed]
- Zhang DF, Teng JF. Direct sequencing of mutations in the copper-transporting P-type adenosine triphosphate (ATP7B) gene for diagnosis and pathogenesis of Wilson's disease. Genet Mol Res 2016;15. [Crossref] [PubMed]
- Alam ST, Rahman MM, Islam KA, et al. Neurologic manifestations, diagnosis and management of Wilson's disease in children - an update. Mymensingh Med J 2014;23:195-203. [PubMed]
- Roberts EA, Schilsky ML. American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: an update. AASLD practice guidelines. Hepatology 2008;47:2089-111. [Crossref] [PubMed]
- European Association for Study of Liver. EASL Clinical Practice Guidelines Wilson’s disease. J Hepatol 2012;56:671-85. [Crossref] [PubMed]
- Brunet AS, Marotte S, Guillaud O, et al. Familial screening in Wilson's disease: think at the previous generation! J Hepatol 2012;57:1394-5. [Crossref] [PubMed]
- Ala A, Borjigin J, Rochwarger A, et al. Wilson disease in septuagenarian siblings: raising the bar for diagnosis. Hepatology 2005;41:668-70. [Crossref] [PubMed]
- Li H, Liu L, Li Y, et al. Familial screening of children with Wilson disease: Necessity of screening in previous generation and screening methods. Medicine (Baltimore) 2018;97:e11405. [Crossref] [PubMed]
- Ala A, Walker AP, Ashkan K, et al. Wilson disease. Lancet 2007;369:397-408. [Crossref] [PubMed]
- Gupta A, Chattopadhyay I, Dey S, et al. Molecular pathogenesis of Wilson disease among Indians: a perspective on mutation spectrum in ATP7B gene, prevalent defects, clinical heterogeneity and implication towards diagnosis. Cell Mol Neurobiol 2007;27:1023-33. [Crossref] [PubMed]
- Lorincz MT. Neurologic Wilson’s disease. Ann N Y Acad Sci 2010;1184:173-87. [Crossref] [PubMed]
- Dzieżyc K, Gromadzka G, Członkowska A. Wilson’s disease in consecutive generations of one family. Parkinsonism Relat Disord 2011;17:577-8. [Crossref] [PubMed]
- Ferenci P. Wilson disease. Clin Gastroenterol Hepatol 2005;3:726-33. [Crossref] [PubMed]
- Gromadzka G, Chabik G, Mendel T, et al. Middle-aged heterozygous carriers of Wilson’s disease do not present with significant phenotypic deviations related to copper metabolism. J Genet 2010;89:463-7. [Crossref] [PubMed]
- Sánchez-Albisua I, Garde T, Hierro L, et al. A high index of suspicion: the key to an early diagnosis of Wilson’s disease in childhood. J Pediatr Gastroenterol Nutr 1999;28:186-90. [Crossref] [PubMed]
- Perman JA, Werlin SL, Grand RJ, et al. Laboratory measures of copper metabolism in the differentiation of chronic active hepatitis and Wilson disease in children. J Pediatr 1979;94:564-8. [Crossref] [PubMed]
- Ferenci P. Whom and How to screen for Wilson disease. Expert Rev Gastroenterol Hepatol 2014;8:513-20. [Crossref] [PubMed]
- Kroll CA, Ferber MJ, Dawson BD. Diagnosis of Wilson Disease in Young Children: Molecular Genetic Testing and a Paradigm Shift from the Laboratory Diagnosis. Mol Genet Metab 2006;89:134-8. [Crossref] [PubMed]
- Tu JB, Blackwell RQ. Studies on levels of penicillamine-induced cupriuresis in heterozygotes of Wilson’s disease. Metabolism 1967;16:507-13. [Crossref] [PubMed]
- Dhawan A, Taylor RM, Cheeseman P, et al. Wilson’s disease in children: 37-year experience and revised King’s score for liver transplantation. Liver Transpl 2005;11:441-8. [Crossref] [PubMed]
- Ludwig J, Moyer TP, Rakela J. The liver biopsy diagnosis of Wilson’s disease. Am J Clin Pathol 1994;102:443-6. [Crossref] [PubMed]
- Martins da Costa C, Baldwin D, Portmann B, et al. Value of urinary copper excretion after penicillamine challenge in the diagnosis of Wilson’s disease. Hepatology 1992;15:609-15. [Crossref] [PubMed]
- Müller T, Koppikar S, Taylor RM, et al. Re-evaluation of the penicillamine challenge test in the diagnosis of Wilson’s disease in children. J Hepatol 2007;47:270-6. [Crossref] [PubMed]
- Steindl P, Ferenci P, Dienes HP, et al. Wilson’s disease in patients presenting with liver disease: a diagnostic challenge. Gastroenterology 1997;113:212-8. [Crossref] [PubMed]
- Roberts EA, Cox DW. Wilson disease. Baillieres Clin Gastroenterol 1998;12:237-56. [Crossref] [PubMed]
- Ferenci P, Steindl-Munda P, Vogel W, et al. Diagnostic value of quantitative hepatic copper determination in patients with Wilson's Disease. Clin Gastroenterol Hepatol 2005;3:811-8. [Crossref] [PubMed]
- Gow PJ, Smallwood RA, Angus PW, et al. Diagnosis of Wilson’s disease: an experience over three decades. Gut 2000;46:415-9. [Crossref] [PubMed]
- Frommer D, Morris J, Sherlock S, et al. Kayse-Fleischer-like rings in patients without Wilson’s disease. Gastroenterology 1977;72:1331-5. [PubMed]
- Fleming CR, Dickson ER, Wahner HW, et al. Pigmented corneal rings in non-Wilsonian liver disease. Ann Intern Med 1977;86:285-8. [Crossref] [PubMed]
- Nagral A, Jhavefi A, Nalawade S, et al. Kayser-Fleischer rings or bile pigment rings? Indian J Gastroenterol 2015;34:410-2. [Crossref] [PubMed]
- Chen C, Shen B, Xiao JJ, et al. Currently Clinical Views on Genetics of Wilson’s Disease. Chin Med J (Engl) 2015;128:1826-30. [Crossref] [PubMed]
- Jesse M, Dempsey R, Eshelman A, et al. Screening for Wilson's disease: which tests are good enough? Liver Transpl 2014;20:1525-6. [PubMed]
- Ferenci P, Caca K, Loudianos G, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int 2003;23:139-42. [Crossref] [PubMed]
- Motamed F, Benabbas R, Ashrafi MR, et al. Ataxia-telangiectasia or neurologic Wilson's disease: when strong family history becomes a diagnostic bias. Acta Neurol Belg 2013;113:195-6. [Crossref] [PubMed]
- Jung S, Whiteaker JR, Zhao L. Quantification of ATP7B protein in Dried Blood Spots by Peptide Immuno-SRM as a potential screen for Wilson Disease. J Proteome Res 2017;16:862-71. [Crossref] [PubMed]


