
Association between carotid plaque and Parkinson’s disease
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders that classically presents with extrapyramidal symptoms and nonmotor symptoms (1). The disease manifests as a resting tremor, with rigidity, bradykinesia, and postural instability, and it can also be accompanied by several other nonmotor symptoms such as muscular pain, anxiety, depression and other disturbances (2). The mutations of some known genes lead to some PD cases; however, this disorder is mostly the result of an interaction with the inherent genetic and environmental effect conditions (3). Both genetic and environmental factors conduce to the pathogenetic process of PD (1). It is characterized by a diminishing of dopaminergic neurons located in the substantia nigra. Several biological processes mechanisms for the degeneration of nigrostriatal neurons are oxidative stress (4), inflammation (5), mitochondrial dysfunction (6) and protein aggregation (7), with oxidative stress and mitochondrial dysfunction being considered as playing the most significant roles. Urate is a robust endogenous antioxidant, serum uric acid (UA), in particular, has recently drawn extensive clinical attention. There are plenty of studies which have revealed that high levels of serum or plasma urate reduced the risk of PD (8-10). Ascherio et al. showed that he higher serum and cerebrospinal fluid urate concentrations at baseline could be slowed down in the progression of PD, implying a potential neuroprotection with a high level of urate in PD patients (11). Meanwhile, another study found that the elevation of serum UA was associated with the occurrence and development of PD. The patients with a low level of serum UA may easily develop PD (12). Moreover, other research (13-15) has also suggested the effect of lipid and cholesterol metabolism in the pathogenesis of PD. Serum cholesterol is a crucial, decisive factor of serum coenzyme Q10 levels, while coenzyme Q10 works as a powerful mitochondrial electron and antioxidant acceptor, and can lead to beneficial effects in PD patients (16).
Hyperlipidemia and hyperuricemia are independent risk factors for cardiovascular disease. The Kumral study (17) found that a higher level of UA and carotid artery disease (CAD) are closely interrelated. Lectin-like oxidized low-density lipoprotein 1(LOX1) may be one of the non-neglected factors during the formation of vulnerable carotid plaque (18). Struck et al. (19), and other professors (20) have reported that, in PD patients, the UA and LDL-C were significantly lower in the PD group than those in healthy, people and the risk of stroke decreased. Also, one study by Korten et al. found that among 1,516 stroke patients, there were only eight PD individuals (21). However, up to now, the study of the carotid plaque of PD patients still has not been clarified. Therefore, a retrospective study focussing on the carotid plaque of PD patients and the relationship between plaque with UA and LDL-C was carried out.
Methods
A total of 68 patients who were diagnosed by the UK PD Society Brain Bank criteria (22) in Jiangsu Sheng hospital between January 2010 and October 2017. We excluded patients with Parkinsonism syndrome, Parkinsonism plus syndrome, severe heart and cerebrovascular disease, and liver and kidney disease. Meanwhile, the control group consisted of 81 healthy people, who were at the same age period and gender as the participants in the PD group. The study was approved by the Ethics Committee of the Nanjing Medical University (JSSZYYLL-20181126-01), and written informed consent was obtained from all the participants.
The 5-mL fasting blood of the ulnar vein was taken from all subjects who had stopped their high-fat and high-purine diet a day before. The content of UA and LDL-C were determined by the Roche P800 fully automatic biochemical analyzer. Each PD patient was evaluated according to the following H&Y stages: (I) unilateral involvement usually only with minimal or no functional disability; (II) bilateral or midline involvement without impairment of balance; (III) bilateral disease of mild to moderate disability with impaired postural reflexes but physically independent; (IV) Severely disabling disease with the ability to still walk or stand unassisted; (V) confinement to bed or wheelchair unless otherwise aided.
Using ultrasound, the vascular morphologic changes, endothelial function, and the echo of plaques were measured in the common carotid artery (CCA), the bifurcation of CCA, and the proximal segment of the unilateral internal carotid artery (ICA). The built-in software of the ultrasound system was used to calculate the total intima-media surface of this selected area online. The definition of carotid plaques is thickened walls of local blood vessels, and consists of any one of the following criteria: the intima-media thickness (IMT) being ≥1.3 mm, the limitations bulge protruding into the lumen ≥2.5 mm, or the IMT thickened more than 50% of the surrounding tissue (23). We define the uniformly, predominantly and mixed echo lucent as the unstable plaques, while the predominantly and uniformly echogenic plaques as the stable plaques.
All the statistical analyses were performed with the SPSS 22.0 for Windows. The measurement data were presented as mean ± SD, while the t-test was used to compare the two independent sample means, and the chi-square test was used for the comparison of enumeration data. The differences of mean among the three groups were determined with one-way analysis of variance. The Pearson and Spearman correlation analysis were used to test the differences in IMT between groups holding consistent age courses of the disease and the illness severity. The statistical significance was set at P≤0.05 level.
Results
Table 1 shows the demographics, the history of hypertension, smoking status, diabetes and the use of alcohol in both groups. There were no significant differences between the two groups in gender, age, history of hypertension, diabetes mellitus and the use of alcohol.

Full table
Among the total of 68 patients in the PD group, there were 38 males and 30 females, aged 39–86 (72.41±9.27) years. The time of disease was 0.1–10 (3.26±2.58) years. The PD group had the following H&Y stage distribution: 33 cases were stage 1 to 2, 26 cases were stage 2.5 to 3, and 9 cases were above 3 stage. Forty-four males and 37 females were in the control group, and the mean age was 71.60±9.70 years.
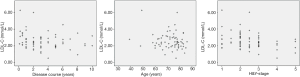
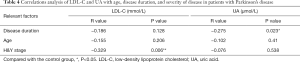
As shown in Tables 2,3, in the PD group, the serum UA level was 282.38±92.81 µmol/L, while in the control group the serum UA level was 331.07±96.51 µmol/L. There was a significant difference in serum levels of UA for the two groups (P=0.002). PD patients had a significantly lower serum UA level than the control subjects. In the PD group, the serum LDL-C level was 2.57±0.98 mmol/L, while in the control group the serum LDL-C level was 2.96±0.75 mmol/L. There was a significant difference in serum levels of LDL-C for the two groups (P=0.008). PD patients had a significantly lower serum LDL-C level than the control subjects. However, 33 of PD patients were detected the carotid plaques, and 46 subjects in the control group had carotid plaques. Neither the mean of the IMT of the CCA nor the incidence of carotid plaque demonstrated any difference in the two groups. As for three subgroups of the Parkinson’s patients, we observed the level of LDL-C and UA. We found that there were no significant differences among these subgroups.

Full table

Full table
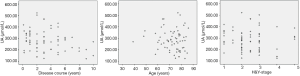
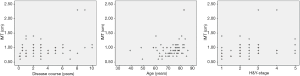
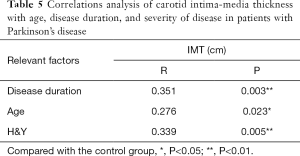
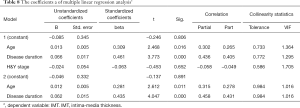
Pearson correlation analysis showed a significant correlation between LDL-C and H&Y (P=0.006<0.01), and there was a strong association between UA and disease duration (P=0.023<0.05) (Figures 1,2 and Table 4). IMT was associated with age, disease course, and severity of the disease (Figure 3 and Table 5). Multiple linear regression analysis predicting the relationship among variables showed that IMT had a significant association with age and disease duration (Tables 6-8).
Full table
Full table
Full table
Full table
Full table
Discussion
This study shows that UA and LDL-C in Parkinson’s patients were significantly lower than in the control group. However, there was no significant difference between carotid IMT and plaque incidence rate. Patients with PD were divided into three subgroups, and the results showed that there were no significant differences among the three groups. Pearson correlation analysis showed a significant correlation among IMT and age, duration of disease and H&Y stage.
We found significantly lower LDL-C in cases that developed PD, compared with controls. These results were consistent with previous studies. For example, one study suggested that a high level of total cholesterol increased the risk of PD (24). The study by Scigliano et al. found that the level of TG was significantly lower in PD patients than in control patients who had other non-cerebrovascular neurological diseases (14). A retrospective study found that the serum levels of TG, VLDL-C, and apoB were significantly lower in the PD group than in any other groups with non-cerebrovascular neurological diseases, acute intracerebral hemorrhage and acute cerebral infarction (25). Many environmental risk factor studies corroborated the involvement of insufficient lipid and lipoprotein in the pathophysiology of PD. As described above, high levels of plasma total cholesterol are related to a lower risk of PD.
Nevertheless, contrary results were also found. Vikdahl (26) found that there were no differences in serum cholesterol levels between cases and controls. Higher intake of polyunsaturated fatty acid might be inversely associated with PD risk (27). Other studies showed that low levels of plasma LDL were associated with increased PD risk (13,28), whereas high levels of plasma HDL and CSF oxysterol were linked to a higher PD risk and duration (29,30). Finally, in PD patients, high levels of sphingolipids, oxysterols, and oxidized LDL were found in the plasma (31,32).
Given an aging society, more and more carotid plaque is found with the increasing use of statins, and it is important to determine whether long-term statin usage may also lead to a higher PD risk by lowering plasma cholesterol levels. Huang et al. reported that statin use could increase the risk of PD, and higher levels of plasma total or LDL cholesterol were associated with a lower risk of PD (33). It is well-established that serum cholesterol is closely related to coenzyme Q10, which itself acts as a potent endogenous antioxidant, and, as a vital electron acceptor, plays a role in the mitochondrial respiratory chain. Coenzyme Q10 and cholesterol are derived from the same biosynthetic pathway; in fact, all of the coenzyme Q10 in plasma is incorporated in lipoproteins, primarily the LDL-C (34), and thus, coenzyme Q10 could be a possible therapy for PD.
In this study, the levels of serum UA in PD were significantly lower than those in control subjects (Table 2), and this result was consistent with previous findings (35,36) showing that lower UA levels may lead to exacerbating PD as a result of oxidative stress in PD (37). The inverse relationship between UA and severity of PD is different for males and females (12). Whether in vivo or in vitro in PD models, the protective effect on nigral dopaminergic neurons has been confirmed (38,39).
Higher UA level is demonstrated to be strongly associated with CAD. The higher UA level is significantly associated with the pathological changes in the carotid artery. In addition, IMT of the bifurcation to hyperuricemia which indicates the higher UA level as an accelerating agent, acts on the process of carotid atherosclerosis (17). It is also known that high plasma levels of lipids and lipoproteins are associated with stroke (40). LDL-C was independently associated with atherosclerosis presence and extent (41). Some studies have found that long-term application of levodopa in the treatment of PD can cause plasma Hcy to increase (42). Elevated plasma Hcy may lead to carotid artery thickening and be involved in carotid plaque formation (43).
However, in our study, there were no differences in IMT and plaque between the PD group and the control group. Even in the subgroups, there was no significant difference in the level of LDL-L and UA. These results indicated that some other factors take part in the process of atherosclerosis. Therefore, we analyzed the correlation between the LDL-C, UA, age, duration of disease and H&Y. There was a significant correlation between LDL-C and the H&Y, while UA was related to the length of disease. IMT correlated with age, duration of disease and H&Y, so we performed multiple linear regression analysis and found that age and duration of disease were strictly related to IMT. Serum levels of LDL-C were inversely correlated with H&Y in PD patients, while UA was related to disease progression. In PD patients, like other diseases, age contributes to the process of IMT; at the same time, disease progress also plays a role in the carotid artery arteriosclerosis, although we do not know the exact mechanism.
In conclusion, we found that there were no differences in carotid artery arteriosclerosis plaque and IMT, but the PD progress indeed was correlated with IMT. LDL-C and UA, to some extent, have different priorities with H&Y and disease progression. As for the exact mechanism, unfortunately, we did not have specific and accurate answers.
Using only cross-sectional studies, and small sample sizes is a deficiency in our study. Further prospective studies are needed to confirm the results. Because of these deficiencies, our research only could reach preliminary conclusions.
Acknowledgements
Funding: This work was supported by the Suzhou Science and Technology Development Project (grant No. SYSD205046).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of the Nanjing Medical University (JSSZYYLL-20181126-01), and written informed consent was obtained from all the participants.
References
- Pringsheim T, Jette N, Frolkis A, et al. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2014;29:1583-90. [Crossref] [PubMed]
- Gallegos S, Pacheco C, Peters C, et al. Features of alpha-synuclein that could explain the progression and irreversibility of Parkinson's disease. Front Neurosci 2015;9:59. [Crossref] [PubMed]
- Tanner CM. Is the cause of Parkinson's disease environmental or hereditary? Evidence from twin studies. Adv Neurol 2003;91:133-42. [PubMed]
- Blesa J, Trigo-Damas I, Quiroga-Varela A, et al. Oxidative stress and Parkinson's disease. Front Neuroanat 2015;9:91. [Crossref] [PubMed]
- Chen H, Mosley TH, Alonso A, et al. Plasma urate and Parkinson's disease in the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2009;169:1064-9. [Crossref] [PubMed]
- Ryan BJ, Hoek S, Fon EA, et al. Mitochondrial dysfunction and mitophagy in Parkinson's: from familial to sporadic disease. Trends Biochem Sci 2015;40:200-10. [Crossref] [PubMed]
- Ebrahimi-Fakhari D, Wahlster L, McLean PJ. Protein degradation pathways in Parkinson's disease: curse or blessing. Acta Neuropathol 2012;124:153-72. [Crossref] [PubMed]
- Weisskopf MG, O'Reilly E, Chen H, et al. Plasma urate and risk of Parkinson's disease. Am J Epidemiol 2007;166:561-7. [Crossref] [PubMed]
- de Lau LM, Koudstaal PJ, Hofman A, et al. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 2005;58:797-800. [Crossref] [PubMed]
- Davis JW, Grandinetti A, Waslien CI, et al. Observations on serum uric acid levels and the risk of idiopathic Parkinson's disease. Am J Epidemiol 1996;144:480-4. [Crossref] [PubMed]
- Ascherio A, LeWitt PA, Xu K, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol 2009;66:1460-8. [Crossref] [PubMed]
- Pan M, Gao H, Long L, et al. Serum uric acid in patients with Parkinson's disease and vascular parkinsonism: a cross-sectional study. Neuroimmunomodulation 2013;20:19-28. [Crossref] [PubMed]
- Huang X, Chen H, Miller WC, et al. Lower low-density lipoprotein cholesterol levels are associated with Parkinson's disease. Mov Disord 2007;22:377-81. [Crossref] [PubMed]
- Scigliano G, Musicco M, Soliveri P, et al. Reduced risk factors for vascular disorders in Parkinson disease patients: a case-control study. Stroke 2006;37:1184-8. [Crossref] [PubMed]
- Miyake Y, Tanaka K, Fukushima W, et al. Case-control study of risk of Parkinson's disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J Neurol Sci 2010;293:82-6. [Crossref] [PubMed]
- Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol 2002;59:1541-50. [Crossref] [PubMed]
- Kumral E, Karaman B, Orman M, et al. Association of uric acid and carotid artery disease in patients with ischemic stroke. Acta Neurol Scand 2014;130:11-7. [Crossref] [PubMed]
- Saito A, Fujimura M, Inoue T, et al. Relationship between lectin-like oxidized low-density lipoprotein receptor 1 expression and preoperative echogenic findings of vulnerable carotid plaque. Acta Neurochir (Wien) 2010;152:589-95. [Crossref] [PubMed]
- Struck LK, Rodnitzky RL, Dobson JK. Stroke and its modification in Parkinson's disease. Stroke 1990;21:1395-9. [Crossref] [PubMed]
- Nataraj A, Rajput AH. Parkinson's disease, stroke, and related epidemiology. Mov Disord 2005;20:1476-80. [Crossref] [PubMed]
- Korten A, Lodder J, Vreeling F, et al. Stroke and idiopathic Parkinson's disease: does a shortage of dopamine offer protection against stroke. Mov Disord 2001;16:119-23. [Crossref] [PubMed]
- Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181-4. [Crossref] [PubMed]
- Naik V, Gamad RS, Bansod PP. Carotid artery segmentation in ultrasound images and measurement of intima-media thickness. Biomed Res Int 2013;2013:801962. [Crossref] [PubMed]
- Hu G, Antikainen R, Jousilahti P, et al. Total cholesterol and the risk of Parkinson disease. Neurology 2008;70:1972-9. [Crossref] [PubMed]
- Wei Q, Wang H, Tian Y, et al. Reduced serum levels of triglyceride, very low-density lipoprotein cholesterol and apolipoprotein B in Parkinson's disease patients. PLoS One 2013;8:e75743. [Crossref] [PubMed]
- Vikdahl M, Bäckman L, Johansson I, et al. Cardiovascular risk factors and the risk of Parkinson's disease. Eur J Clin Nutr 2015;69:729-33. [Crossref] [PubMed]
- Wang A, Lin Y, Wu Y, et al. Macronutrients intake and risk of Parkinson's disease: A meta-analysis. Geriatr Gerontol Int 2015;15:606-16. [Crossref] [PubMed]
- Huang X, Abbott RD, Petrovitch H, et al. Low LDL cholesterol and increased risk of Parkinson's disease: prospective results from Honolulu-Asia Aging Study. Mov Disord 2008;23:1013-8. [Crossref] [PubMed]
- Cassani E, Cereda E, Barichella M, et al. Cardiometabolic factors and disease duration in patients with Parkinson's disease. Nutrition 2013;29:1331-5. [Crossref] [PubMed]
- Björkhem I, Lövgren-Sandblom A, Leoni V, et al. Oxysterols and Parkinson's disease: evidence that levels of 24S-hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci Lett 2013;555:102-5. [Crossref] [PubMed]
- Andican G, Konukoglu D, Bozluolcay M, et al. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson's disease. Acta Neurol Belg 2012;112:155-9. [Crossref] [PubMed]
- Mielke MM, Maetzler W, Haughey NJ, et al. Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson's disease and associated with cognitive impairment: a pilot study. PLoS One 2013;8:e73094. [Crossref] [PubMed]
- Huang X, Alonso A, Guo X, et al. Statins, plasma cholesterol, and risk of Parkinson's disease: a prospective study. Mov Disord 2015;30:552-9. [Crossref] [PubMed]
- Miles MV, Horn PS, Morrison JA, et al. Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin Chim Acta 2003;332:123-32. [Crossref] [PubMed]
- Fitzmaurice PS, Ang L, Guttman M, et al. Nigral glutathione deficiency is not specific for idiopathic Parkinson's disease. Mov Disord 2003;18:969-76. [Crossref] [PubMed]
- Church WH, Ward VL. Uric acid is reduced in the substantia nigra in Parkinson's disease: effect on dopamine oxidation. Brain Res Bull 1994;33:419-25. [Crossref] [PubMed]
- Winquist A, Steenland K, Shankar A. Higher serum uric acid associated with decreased Parkinson's disease prevalence in a large community-based survey. Mov Disord 2010;25:932-6. [Crossref] [PubMed]
- Guerreiro S, Ponceau A, Toulorge D, et al. Protection of midbrain dopaminergic neurons by the end-product of purine metabolism uric acid: potentiation by low-level depolarization. J Neurochem 2009;109:1118-28. [Crossref] [PubMed]
- Duan W, Ladenheim B, Cutler RG, et al. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease. J Neurochem 2002;80:101-10. [Crossref] [PubMed]
- Benson RT, Sacco RL. Stroke prevention: hypertension, diabetes, tobacco, and lipids. Neurol Clin 2000;18:309-19. [Crossref] [PubMed]
- Fernández-Friera L, Fuster V, López-Melgar B, et al. Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors. J Am Coll Cardiol 2017;70:2979-91. [Crossref] [PubMed]
- Yuan RY, Sheu JJ, Yu JM, et al. Methylenetetrahydrofolate reductase polymorphisms and plasma homocysteine in levodopa-treated and non-treated Parkinson's disease patients. J Neurol Sci 2009;287:64-8. [Crossref] [PubMed]
- Dietrich M, Jacques PF, Polak JF, et al. Segment-specific association between plasma homocysteine level and carotid artery intima-media thickness in the Framingham Offspring Study. J Stroke Cerebrovasc Dis 2011;20:155-61. [Crossref] [PubMed]


