
Extracorporeal radiation and reimplantation: a safe and viable option for reconstruction after sacral tumor resection?
Introduction
Reconstruction after total sacrectomy for primary bone tumors is complex, involving mechanical, biological, and soft tissue reconstruction while achieving adequate functional and oncological outcomes (1-3). Mechanical reconstruction of the pelvic ring traditionally utilizes a spino-pelvic construct (4) and incorporates biological reinforcement with structural autograft (most commonly fibula, free or vascularized) or allograft (fresh frozen or irradiated, commonly used are fibula and femur) (3). More recently some surgeons have used custom 3D sacral implants to restore the spino-pelvic anatomy (5). Finally, the posterior soft tissue reconstruction is typically done via the vertical rectus abdominis myocutaneous (VRAM) flap (6). The lead author on this report (MLG) traveled to live in Mumbai, India for one month to learn from the senior author (MA) and his team. One of the most striking differences in surgical approaches to sacro-pelvic tumors was their use of extracorporeal radiation therapy (ECRT), whereby the patient’s tumor is removed en bloc, sent for ex vivo radiation therapy, then re-implanted to serve as a perfectly-matched bone graft (7-9). Given the early success with this technique and numerous requests about it (particularly from countries where this is not utilized (e.g., USA), we present here a short case report and technical description describing our methods.
Illustrative case
A 45 years old man presented with 2 months of back pain, left-sided radicular pain, and urinary incontinence. Imaging revealed a large sacral mass that extended as high as S1 and bilaterally, abutting but not traversing the SI joints (Figure 1). An image-guided core needle biopsy revealed a grade 2 myxoid chondrosarcoma. After staging revealed no other sites of disease, the patient was indicated for total sacrectomy. The patient was counseled regarding the magnitude of surgery necessary for complete tumor clearance, including ligation of the thecal sac below the L5 level along with the total sacrectomy. The patient understood that surgery would come with complete loss of motor and sensory function in S1 roots and below, urinary incontinence requiring self-catheterization, and a permanent diverting colostomy. Reconstruction for the bony defect was planned (ECRT and sacro-pelvic fixation to complete the pelvic ring and allow transmission of forces from pelvis onto the spine), as was reconstruction for the soft tissue defect [transposition of the anterior vertical rectus abdominis musculocutaneous (VRAM) flap posteriorly].

Procedure
Stage 1: VRAM harvest, L5/1 discectomy, and bone cuts
A standard time-out to identify the patient and procedure was performed and all parties agreed. The standard vertical incision was used to harvest the VRAM flap in the usual method (Figure 2A,B). A diverting colostomy was also uneventfully performed at this time. After the approach was performed, tumor bulge on the left side of anterior sacrum was easily visible. Given lack of efficacious treatment options outside of surgery for chondrosarcoma, care was taken throughout not to disturb the tumor bed. Major iliac vessels were protected, and the L5/S1 discectomy was performed uneventfully. Additionally, the sacroiliac (SI) joints were identified and bone cuts were made just lateral to the joint bilaterally. Mesh was used to close the abdomen (Figure 2C); skin was closed uneventfully. The patient remained intubated and was then positioned prone.
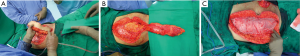
Stage 2: prone sacrectomy and ECRT
Once prone, a midline incision was made from around L3-coccyx (Figure 3A). After securing appropriate exposure to the iliac wings, L5/S1 was decompressed and the thecal sac tied off (Figure 3B). Bilateral S1 roots were identified and ligated. Posterior cuts lateral to the SI joint were made to join with the previous anterior cuts. An osteotomy cut was made through the sacro-coccyx joint, and all attachments to the sacrum was safely transected with margins. At this point the S1-3 roots were ligated as the sacrum was resected. The specimen was carefully removed, and it was placed on the back table, where it was then prepared to go for extracorporeal radiation therapy (ECRT) (Figure 3C,D,E,F,G).
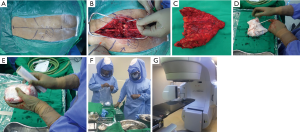
On the back table, the specimen was carefully inspected, however no sample of tumor was taken at this time as to avoid contamination of the surgical field. After soaking the resected specimen in vancomycin solution (2 g vancomycin/1 L saline) (8) a sponge from the surgical field was accounted for and wrapped around the specimen (2 if needed). Next, sterile plastic wrap was then tightly wrapped around the specimen in multiple layers (≈15) to create a sealed packaging with no trapped air pockets. It was then placed in one more outer impervious wraps before being placed in a sealed bag. It was then passed off the surgical field, where it was carefully transported to the radiation suite for radiation therapy. The specimen then received 50 Gy single fraction irradiation using the linear accelerator.
While the removed bone was undergoing radiation, hemostasis was achieved and the wound was washed thoroughly. Once the tumor returned from ECRT (typically ≈45 min in our experience), it was placed on a new sterile back table and carefully unwrapped. The radiated bone was then stripped of soft tissues, and the tumor areas carefully scraped out using rongeurs, curettes, and scalpel as needed. The debulked tumor tissue was sent for histopathology. Once dead tumor tissue was debrided (Figure 4A,B), the specimen was carefully rinsed and the process repeated (Figure 4C). This was continued until all cartilage, dead soft tissue, and previous tumor tissue were removed. Care was taken at this time not to be too aggressive as to remove excess bone. As the aim of reconstruction was to complete the pelvic ring and provide support, excess distal sacrum/coccyx was removed. This distal bone is significantly less useful for structural support and removal of this excess bone allows space for transposing the VRAM flap posteriorly (Figure 4D). Once this was done bone cement (Palacos©) was used to fill the tumor defect area and lend support (Figure 4E). After the bone cement hardened the graft was soaked in a fresh solution of vancomycin before re-implantation.
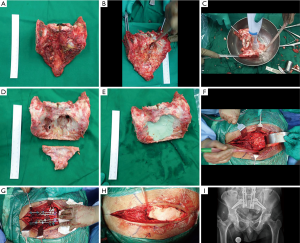
The specimen was then placed into the defect after identification of the VRAM flap and appropriate positioning of it. Two iliac bolts traversing from each posterior ilium into the supracetabular region were placed with petal in-line with the lumbar facets/pedicles. Next, pedicle screws were placed at L3-5 in standard fashion. Once the specimen was in place and secured, rods were placed connecting the pelvic bolts to the lumbar pedicle screws (Figure 4F,G). A standard mesh Harms cage was cut to the desired length and placed in the new L5/S1 disc space, with gentle compression across the rods. Bilateral rods were then placed with connectors with a cross link between to complete the “quad-rod” construct. After final tightening the implants and checking all hardware under fluoroscopy, a thorough wash was performed. The prepared VRAM flap was then used in the standard fashion to assist in closing the wound (Figure 4H,I).
Complication
On post-operative imaging the gap between bone cuts on the left side was wider than anticipated, so a second procedure was performed percutaneously. Two 120 mm × 6.5 mm partially threaded, cannulated screws were successfully placed using a guidewire from iliac to sacrum under CT guidance to close this gap (Figure 5A,B). Postoperatively the patient did well and was discharged to home on POD17. He had incontinence with the need for self-catheterization (as expected) and maintained good care of his diverting colostomy. He also maintained good lower extremity motor function and was able to stand and walk daily.
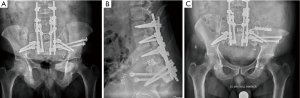
The patient did well in this case until almost one year out from surgery. At that point he had developing pain near his SI joints. CT at the time showed poor fusion across the osteotomies, and the patient was taken back for bilateral osteotomy site autografting, after which he did well (Figure 5C).
Conclusions/discussion
First reported in 1968 by Spira and Lubin (7), ECRT has been performed relatively few times over the ensuing 50 years. However, recently there has been an increase in interest in this technique in orthopedic oncology as limb-sparing techniques have increased in popularity (8,10,11). Traditional spinopelvic constructs employ non-anatomical structural autografts/allografts, or non-biological anatomical custom-printed 3D sacral prostheses. ECRT was selected as the method of choice for this sacral reconstruction to achieve optimal anatomical, mechanical, and biological restoration of spino-pelvic continuity.
Multiple factors favor use of ECRT over allograft: (I) Some Asian and African and other cultures forbid allograft (12,13), (II) there is risk of disease transmission with allograft not present with ECRT (14-17), (III) maintaining a bone bank can be beyond the budget/infrastructure of many countries (18,19), and (IV) getting perfect fit of allograft, both morphologically and immunologically, to native host bone can be challenging (15).
Techniques that involve re-implantation of the patient’s own bone avoid the problems associated with harvesting autograft or procuring structural allografts and allow for a perfectly-fitted size-matched structural graft. While different techniques have been employed for this (e.g., autoclaving, liquid nitrogen, pasteurization), ECRT has proven to be one of if not the best of these (18,20). Custom or 3D-printed prostheses can also be used for reconstruction, but this is much more expensive and carries its own risks (5); ECRT is a low-cost alternative to these. Finally, the senior author (MA) has substantial experience with this procedure, having successfully used this technique for tumor surgery of the limbs and pelvis in the past (8,9).
Here we add to the very few reports on ECRT by presenting a 45 years old man with chondrosarcoma who underwent sacrectomy with ECRT and reimplantation (7,20,21). Although he remains cancer free and without infection, he did require bone grafting to aid fusion. While not a perfect technique, many of the prior concerns that surround it have been convincingly answered in prior studies (18,20). And while data sets remain relatively small, there has not been an increase in recurrence rates within the ECRT bone, further underscoring its safety for re-implantation (9,12,13,22,23). Hatano et al. (24) carefully analyzed histopathology and determined that a single radiation dose of 60 Gy was sufficient to kill all tumor cells. Several other groups have now reported on radiation dose and use of ECRT (11,18,25,26); it appears that a single dose of 50 Gy is sufficient to eradicate any tumor cells, yet still low enough as to not significantly weaken the bone structure or decrease chance of fusion (27-29). It is worth noting that the irradiation dose that an allograft may undergo is significantly higher (≈3 orders of magnitude!) than the radiation dosing used in ECRT (kGy for allografts vs. Gy for ECRT) (14,30). Structural and biomechanical changes seem to take place at this much higher allograft radiation dosing (14,30), while most properties are maintained at the lower ECRT dosing (25,29). This larger irradiation dose for allografts is used principally to prevent disease transmission.
Large, fresh-frozen allografts used in tumor patients have notoriously high infection rates, on the order of ≈12% (15). The authors of a large retrospective study of fresh-frozen grafts used in tumor surgery out of Harvard-MGH proposed that a large part of this is due to immunological reasons. They conclude by noting that matching a large, structural allograft by size, shape, and major histocompatibility complex (MHC) would be incredibly difficult to achieve (15). When the same group examined 5 years of irradiated graphs, it was noted that the irradiated graphs had higher rates of fracture, a not inconsequential complication (31), but no higher rate of nonunion. ECRT helps solve these problems, creating a perfectly matched graft for size, shape, and MHC, while undergoing ≈1,000-fold lower radiation dose than an irradiated allograft, thus preserving critical bone properties.
Still, concern continues to center around the integrity of the graft and the potential for radiation to weaken the bone or decrease fusion rates. In 2015, Gupta et al. (25) investigated the effect of the dosing of irradiation on the mechanical properties of the bone in an ex vivo model (i.e., removing bone, subjecting it to irradiation, then performing ex vivo biomechanical testing). Remarkably, radiation doses up to 300 Gy had little to no mechanical impact on the structural characteristics of the bone. To evaluate fusion potential, Sabo et al. (29) used a canine model to demonstrate excellent boney fusion rates in the ECRT group (that did not differ from the control group with reimplantation of non-irradiated bone). While the dog model nicely approximates humans (32), 25 Gy instead of 50 Gy was used.
In 2015, Nishizawa et al. (21) published a case report of a patient that underwent ECRT for a sacral chondrosarcoma. To our knowledge, this is the only other report on ECRT in the sacrum for malignancy. While malignancies of the sacrum remain particularly difficult to manage, surgery remains the main line of treatment for primary tumors like chordoma and chondrosarcoma. Despite initial concerns, ECRT has proven to be a safe and viable option for sacral reconstruction after tumor resection. From the Latin os sacrum, translated roughly as “sacred bone”, the sacrum indeed holds a unique position in the human body, providing the connection from the axial to the appendicular skeleton, maintaining integrity of the pelvic ring, and allowing passage of sensitive nerve roots. While resection of some or all of the sacrum carries significant morbidity, primary tumors of the sacrum often require surgery as a life-saving measure. ECRT presents a safe, affordable, and efficacious option for reconstruction. While early results with ECRT in the sacrum are promising, future larger studies should be carried out to track long-term outcomes and help further define any complications.
Acknowledgments
Special thanks to Drs. Kevin B. Jones, David L. Rothberg, Alan K. Stotts, and Charles L. Saltzman of the University of Utah for allowing lead author (ML Goodwin) time away to pursue this elective fellowship in Mumbai, India with Dr. Agarwal.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- van Wulfften Palthe ODR, Houdek MT, Rose PS, et al. How Does the Level of Nerve Root Resection in En Bloc Sacrectomy Influence Patient-Reported Outcomes? Clin Orthop Relat Res 2017;475:607-16. [Crossref] [PubMed]
- Phukan R, Herzog T, Boland PJ, et al. How Does the Level of Sacral Resection for Primary Malignant Bone Tumors Affect Physical and Mental Health, Pain, Mobility, Incontinence, and Sexual Function? Clin Orthop Relat Res 2016;474:687-96. [Crossref] [PubMed]
- Reynolds JJ, Khundkar R, Boriani S, et al. Soft tissue and bone defect management in total sacrectomy for primary sacral tumors: A systematic review with expert recommendations. Spine (Phila Pa 1976) 2016;41:S199-204. [Crossref] [PubMed]
- Kiatisevi P, Piyaskulkaew C, Kunakornsawat S, et al. What Are the Functional Outcomes After Total Sacrectomy Without Spinopelvic Reconstruction? Clin Orthop Relat Res 2017;475:643-55. [Crossref] [PubMed]
- Wei R, Guo W, Ji T, et al. One-step reconstruction with a 3D-printed, custom-made prosthesis after total en bloc sacrectomy: a technical note. Eur Spine J 2017;26:1902-9. [Crossref] [PubMed]
- Houdek MT, Bakri K, Tibbo ME, et al. Outcome and Complications following Vertical Rectus Abdominis Myocutaneous Flap Surgery to Reconstruct Sacrectomy Defects. Plast Reconstr Surg 2018;142:1327-35. [Crossref] [PubMed]
- Spira E, Lubin E. Extracorporeal irradiation of bone tumors. Isr J Med Sci 1968;4:1015-9. [PubMed]
- Agarwal M, Gundavda M, Gupta R, et al. Does extracorporeal irradiation and reimplantation after acetabular resections result in adequate hip function? A preliminary report. Clin Orthop Relat Res 2018;476:1738-48. [Crossref] [PubMed]
- Puri A, Gulia A, Agarwal MG, et al. Extracorporeal irradiated tumor bone: A reconstruction option in diaphyseal Ewing’s sarcomas. Indian J Orthop 2010;44:390-6. [Crossref] [PubMed]
- Tang X. CORR Insights: Does extracorporeal irradiation and reimplantation after acetabulum resections result in adequate hip function? A prelim report. Clin Orthop Relat Res 2018;476:1749-50. [Crossref] [PubMed]
- Wu PK, Chen CF, Chen CM, et al. Intraoperative Extracorporeal Irradiation and Frozen Treatment on Tumor-bearing Autografts Show Equivalent Outcomes for Biologic Reconstruction. Clin Orthop Relat Res 2018;476:877-89. [Crossref] [PubMed]
- Zhang S, Wang X, Wang J, et al. En bloc resection, intraoperative extracorporeal irradiation and re-implantation of involved bone for the treatment of limb malignancies. Mol Clin Oncol 2017;7:1045-52. [PubMed]
- Araki N, Myoui A, Kuratsu S, et al. Intraoperative extracorporeal autogenous irradiated bone grafts in tumor surgery. Clin Orthop Relat Res 1999.196-206. [PubMed]
- Hamer AJ, Strachan J, Black M, et al. Biomechanical properties of cortical allograft bone using a new method of bone strength measurement. J Bone Joint Surg Br 1996;78:363-8. [Crossref] [PubMed]
- Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res 2005.210-6. [Crossref] [PubMed]
- Matejovsky Z, Matejovsky Z, Kofranek I. Massive allografts in tumour surgery. Int Orthop 2006;30:478-83. [Crossref] [PubMed]
- Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am 1995;77:1742-54. [Crossref] [PubMed]
- Yasin NF, Singh VA, Saad M, et al. Which is the best method of sterilization for recycled bone autograft in limb salvage surgery: A radiological, biomechanical and histopathological study in rabbit. BMC Cancer 2015;15:289. [Crossref] [PubMed]
- Tomford WW, Mankin HJ. Bone banking: Update on methods and materials. Orthop Clin North Am 1999;30:565-70. [Crossref] [PubMed]
- Singh VA, Nagalingam J, Saad M, et al. Which is the best method of sterilization of tumour bone for reimplantation? a biomechanical and histopathological study. Biomed Eng Online 2010;9:48. [Crossref] [PubMed]
- Nishizawa K, Mori K, Saruhashi Y, et al. Long-term clinical outcome of sacral chondrosarcoma treated by total en bloc sacrectomy and reconstruction of lumbosacral and pelvic ring using intraoperative extracorporeal irradiated autologous tumor-bearing sacrum: A case report with 10 years follow-up. Spine J 2014;14:e1-8. [Crossref] [PubMed]
- Böhm P, Fritz J, Thiede S, et al. Reimplantation of extracorporeal irradiated bone segments in musculoskeletal tumor surgery: clinical experience in eight patients and review of the literature. Langenbecks Arch Surg 2003;387:355-65. [PubMed]
- Chen WM, Chen TH, Huang CK, et al. Treatment of malignant bone tumours by extracorporeally irradiated autograft-prosthetic composite arthroplasty. J Bone Joint Surg Br 2002;84:1156-61. [Crossref] [PubMed]
- Hatano H, Ogose A, Hotta T, et al. Extracorporeal irradiated autogenous osteochondral graft. J Bone Joint Surg Br 2005;87:1006-11. [Crossref] [PubMed]
- Gupta S, Cafferky D, Cowie F, et al. The mechanical effects of extracorporeal irradiation on bone. Bone Joint J 2015;97-B:1152-6. [Crossref] [PubMed]
- Kotb SZ, Mostafa MF. Recycling of extracorporeally irradiated autograft for malignant bone tumors: Long-term follow-up. Ann Plast Surg 2013;71:493-9. [Crossref] [PubMed]
- Sanjay BKS, Moreau PG, Younge DA. Reimplantation of autoclaved tumour bone in limb salvage surgery. Int Orthop 1997;21:291-7. [Crossref] [PubMed]
- Davidson AW, Hong A, McCarthy SW, et al. En-bloc resection, extracorporeal irradiation, and re-implantation in limb salvage for bony malignancies. J Bone Joint Surg Br 2005;87:851-7. [Crossref] [PubMed]
- Sabo D, Brocai DRC, Eble M, et al. Influence of extracorporeal irradiation on the reintegration of autologous grafts of bone. J Bone Joint Surg Br 2000;82:276-82. [Crossref] [PubMed]
- Currey JD, Foreman J, Laketić I, et al. Effects of ionizing radiation on the mechanical properties of human bone. J Orthop Res 1997;15:111-7. [Crossref] [PubMed]
- Lietman SA, Tomford WW, Gebhardt MC, et al. Complications of irradiated allografts in orthopaedic tumor surgery. Clin Orthop Relat Res 2000.214-7. [Crossref] [PubMed]
- Eitel F, Klapp F, Jacobson W, et al. Bone regeneration in animals and in man. Arch Orthop Trauma Surg 1981;99:59-64. [Crossref] [PubMed]


