
Revisiting AJCC TNM staging for renal cell carcinoma: quest for improvement
Cancer staging is the process of determining the magnitude of the primary tumor and extent of its spread. It predicts prognosis, guides management and treatment decisions including enrollment in clinical trials, and helps formulate follow-up and surveillance plans. Cancer staging facilitates the exchange of information among clinicians and researchers within, and across institutions providing a mechanism for comparison of cases across regions, eras, and treatment modalities. It standardizes cancer nomenclature across the spectrum, which helps investigate changes in cancer incidence, its extent at initial presentation, and impact of various policy and treatment interventions. Therefore, from the perspective of cancer diagnosis, cancer staging is the single most important piece of information for patients, clinicians, researchers, and health policy officials. The American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging is the most commonly used and universally accepted staging system for cancer. It is simple, convenient, concise and reproducible. Since its first publication in 1977, AJCC has undergone various changes and as of January 1st, 2018, its 8th edition is used by practicing clinicians (1).
One of the primary dogmas of the AJCC TNM staging is that a higher stage predicts a worse outcome. A constant effort is being made by various stakeholders to improve the prognostic value of TNM staging. A recent report by Shao and colleagues compared the predictive accuracy of the 8th edition AJCC stage grouping to their proposed modifications in a cohort of data on 2,120 renal cell carcinoma (RCC) patients treated at Fudan University Shanghai Cancer Center (FUSCC) between 2000 and 2015 (2). Results from this study revealed that the 5-year overall survival (OS) for T1-3N1M0 (AJCC stage III) was similar to T4N0M0 (AJCC stage IV), and lower then T3N0M0 (AJCC stage III) (38.1% vs. 36.2% vs. 72.7%) in the FUSCC cohort. Using FUSCC cohort as a training set, validation of their findings was done in the Surveillance, Epidemiology, and End Results (SEER) RCC cohort, which included data from 74,506 patients collected between 2004 and 2014, and yielded similar results. Therefore, based on overall survival results, the authors propose to reclassify T1N0M0 and T2N0M0 as stage I, T3N0M0 as stage II, T1-3N1M0 and T4N0M0 as stage III, and T4N1M0 and TanyNanyM1 disease as stage IV. Table 1 provides a tabular view of the sixth, seventh and eight editions of TNM staging and its comparison to proposed modifications.
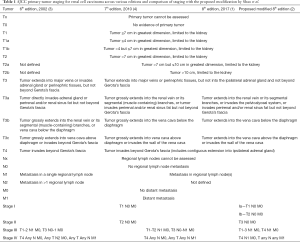
Full table
These modifications, if accepted, will not change the current treatment recommendations, which currently depend on whether the disease is resectable or not. For resectable disease, the standard of care is surgery (partial or radical nephrectomy) while a selected few cases can have the option of active surveillance or ablation. For non-resectable disease, the main treatment options include clinical trials, or systemic agents (immunotherapy and/or targeted therapy) with a potential role for cytoreductive nephrectomy with or without metastasectomy, or radiation and ablative procedures in carefully selected patients (5). However, these modifications may improve prognostic assessment during patient counseling, patient selection for adjuvant therapy trials, and may even aid in designing future adjuvant or neo-adjuvant therapy trials by providing more accurate estimates on survival for a given stage.
The study by Shao et al. (2) had several strengths including its large sample size, statistical design, long follow-up period, validation in the SEER cohort, and sensitivity analysis with stratification for histopathological subtypes, race and years of diagnosis. This study also had limitations such as its retrospective nature and the possibility of inaccurate data entry in the SEER cohort. It should be noted that patients in the SEER database classified as T3a by the 6th AJCC Staging System were excluded in their study since it included patients in whom tumor directly invaded the ipsilateral adrenal gland, which was later classified as T4 disease in the subsequent editions of the AJCC Staging System (Table 1).
Ideally, survival rates between TNM groups should be considerably different between groups and similar within group (good discrimination) (6). Discrimination can be measured by a C statistic or D statistic (in the case of survival outcomes). The C statistic, or concordance index, corresponds to the area under the receiver operating characteristic (ROC) curve for the case of binary outcomes and is a measure of a given prediction model’s ability to discriminate between those with or without the outcome (6). A value of 1 for the C statistic indicates perfect prediction, while a value of 0.5 means that the model is no better than random chance (6). Notably, the concordance index generalizes naturally to time-to-event outcomes, which are commonly subject to right censoring (7). The D statistic is another measure of discrimination, or separation, for time-to-event outcomes (6), and a higher value of the D statistic indicates better discrimination (6). Given that TNM staging places patients in groups of risk relative to other TNM staging groups, calibration, or how closely predicted and actual risk agree, is less relevant as a measure of predictive quality. TNM staging for RCC does not take multiple other important predictive and prognostic factors into consideration (8). Moreover metastatic RCC has shown to have wide inter and intratumor heterogeneity which may cause some patients to have an indolent course while others to have an aggressive disease (9,10).
The study by Shao et al. showed that the predictive discrimination with respect to overall survival in the modified groupings (stages II–IV) was significantly better than current AJCC grouping in both the FUSCC cohort (C-index: 0.801 vs. 0.779, P<0.001) and SEER cohort (C-index: 0.770 vs. 0.764, P<0.001) (2). However, there remains significant room for improvement in the model since the C statistic value is still far from perfect score of 1.
The lack of good discrimination is not just limited to RCC and has led to a never-ending quest to improve the staging system for all cancers. Since its first publication in 1977, AJCC is revising the Cancer Staging Manual every 6 to 8 years in collaboration with the Union for International Cancer Control. However, our understanding of cancer biology and the development of effective treatments is proceeding at a much faster pace. Therefore, several researchers have looked beyond TNM staging to improve prognostic information. In RCC, TNM staging includes multiple important anatomic prognostic factors like tumor size, extension into veins or perinephric tissues, invasion into ipsilateral adrenal gland, or Gerota’s fascia (T), metastasis to regional lymph nodes (N) or distant sites (M) (1). Currently, many novel prognostic markers which include demographic, histologic, clinical, laboratory, and molecular features have been proposed (8,10-13).
Using these markers, multiple prognostic models and nomograms have been proposed. A quick search on PubMed for prognostic models and nomograms in RCC retrieves more than 500 articles. This endless list of proposed models, most of which have not undergone external validation, leads to confusion regarding their applicability and reliability (6). It needs to be stressed that the journey of prognostic models does not end with external validation as they need to be tested in impact studies, ideally in a randomized fashion to evaluate whether their implementation actually improves patient outcomes (6). Currently, the most widely used and endorsed prognostic models are: for localized disease, the University of California Integrated Staging System (UISS), Stage, Size, Grade, and Necrosis (SSIGN) Score, and the Karakiewicz nomogram; and for metastatic disease, the Memorial Sloan-Kettering Cancer Centre (MSKCC), and International Metastatic Renal Cancer Database Consortium (IMDC) or Heng’s model (2,8,14). Further efforts for improvement have led to molecular prognostic models such as ClearCode34, CLEAR score, 16-gene assay, etc. (8). Combining molecular markers with anatomic, pathological, or clinical markers have been reported to significantly increase the C statistic (15), and may be the next step to improving predictive and prognostic models in the era of precision medicine.
Unlike the IMDC model, which has been externally validated in multiple trials (10), most of the other published models have serious limitations making them ineligible for impact trials. Usually they are based on retrospective datasets with inherent biases, have not undergone true external validation (16) rather having been tested in just another cohort, and have not been investigated in prospective studies. Many of the novel molecular models are based on small patient numbers or show evidence of overfitting, lack of generalizability, biases in specimen selection, collection, handling, analysis, and rarity of marker validation strategies (8,17,18). Even though Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) were developed to address these deficiencies, poorly designed research studies exploring predictive models or nomograms continue to be reported (19). To make matters more complicated, guidelines are currently lacking with regards to discrimination (C statistic or AUC), and decision curve analysis to guide what statistical values might be considered clinically important. Finally, it should be remembered that any prognostic model should be clinically applicable before it can be analyzed for internal or external validity. If it is unable to be applied in clinical practice it is worthless no matter how good its statistical powers are (20).
Big datasets, registries, and individual participant data provide some unique opportunities to test these prognostic models in large samples (6). Moreover, multiple prognostic models can be tested simultaneously on the same data set to decide which is most accurate and clinically relevant (21). Molecular markers can be analyzed in retrospective or prospective validation studies while following the REMARK checklist (19). To help with the challenges of more accurate and probabilistic individualized outcome prediction, AJCC has published guidelines to evaluate risk models for individualized prognosis for the purpose of granting endorsement for clinical use (22). Ideally, these prognostic models and nomograms should not only have good accuracy (calibration and discrimination) but should also be simple, reproducible, generalizable or transportable, cost-effective and easy to apply in a fast-paced clinic (20).
However, even in these new models and nomograms, TNM classification will continue to serve as an essential backbone. It continues to be the most important prognostic model and any effort to improve it, as done by Shao et al., is a welcome step in the right direction. With the rapid expansion of our understanding regarding tumor biology and precision medicine, an emphasis is being placed on including predictive (which give information about the effect of therapeutic intervention) and prognostic (which provide information about overall cancer outcome) biomarkers in cancer staging models. AJCC has realized this many years ago and beginning with the 6th edition they started adding nonanatomic factors to modify staging (3). Its 8th edition continues to change from a population-based approach to a personalized one (1). However, even with rapid strides in the development of effective treatments, integration of prognostic and predictive biomarkers with TNM staging are lacking in RCC and continue to be a focus of current investigations (1).
Acknowledgements
Funding: Research reported in this publication utilized the Cancer Biostatistics Shared Resource at Huntsman Cancer Institute at the University of Utah and was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA042014.
Footnote
Conflicts of Interest: Neeraj Agarwal reports consultancy to Pfizer, Novartis, Merck, Genentech, Eisai, Exelixis, Clovis, EMD Serono, BMS, Astra Zeneca, Foundation One, Astellas, Ely Lilly, Bayer, Argos, Medivation, Clovis, and Nektar; and research funding to my institution on my behalf from Active Biotech, Astra Zeneca, Bavarian Nordic, BMS, Calithera, Celldex, Eisai, Exelixis, Genentech, GlaxoSmithKline, Immunomedics, Janssen, Medivation, Merck, New link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Sanofi, Takeda, and Tracon. The other authors have no conflicts of interest to declare.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Amin MB, Edge SB, Greene FL, et al. editors. AJCC Cancer Staging Manual. 8th ed. Switzerland: Springer, 2017.
- Shao N, Wang HK, Zhu Y, et al. Modification of American Joint Committee on cancer prognostic groups for renal cell carcinoma. Cancer Med 2018;7:5431-8. [Crossref] [PubMed]
- Greene FL, Balch CM, Haller DG, et al. editors. AJCC Cancer Staging Manual. 6th ed. New York: Springer, 2002.
- Edge SB, Byrd DR, Compton CC, et al. editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010.
- National Comprehensive Cancer Network. NCCN Guidelines Version 2.2019 Kidney Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Retrieved December 24, 2018.
- Riley RD, Ensor J, Snell KI, et al. External validation of clinical prediction models using big datasets from e-health records or IPD meta-analysis: opportunities and challenges. BMJ 2016;353:i3140. [Crossref] [PubMed]
- Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109-23. [Crossref] [PubMed]
- Klatte T, Rossi SH, Stewart GD. Prognostic factors and prognostic models for renal cell carcinoma: a literature review. World J Urol 2018;36:1943-52. [Crossref] [PubMed]
- Ricketts CJ, De Cubas AA, Fan H, et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep 2018;23:313-26.e5. [Crossref] [PubMed]
- Graham J, Heng DYC, Brugarolas J, et al. Personalized Management of Advanced Kidney Cancer. Am Soc Clin Oncol Educ Book 2018.330-41. [Crossref] [PubMed]
- Sun M, Shariat SF, Cheng C, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol 2011;60:644-61. [Crossref] [PubMed]
- Zhang G, Wu Y, Zhang J, et al. Nomograms for predicting long-term overall survival and disease-specific survival of patients with clear cell renal cell carcinoma. Onco Targets Ther 2018;11:5535-44. [Crossref] [PubMed]
- Eggener S. TNM staging for renal cell carcinoma: time for a new method. Eur Urol 2010;58:517-9; discussion 519-21. [Crossref] [PubMed]
- Ljungberg B, Albiges L, Bensalah K, et al. EAU Guidelines on Renal Cell Carcinoma 2018. European Association of Urology Guidelines. 2018 Edition. Arnhem, The Netherlands: European Association of Urology Guidelines Office, 2018.
- Klatte T, Seligson DB, LaRochelle J, et al. Molecular signatures of localized clear cell renal cell carcinoma to predict disease-free survival after nephrectomy. Cancer Epidemiol Biomarkers Prev 2009;18:894-900. [Crossref] [PubMed]
- Sun M, Trinh QD, Karakiewicz PI. Editorial comment. J Urol 2011;186:1777-8. [Crossref] [PubMed]
- Mandrekar SJ, Sargent DJ. Genomic advances and their impact on clinical trial design. Genome Med 2009;1:69. [Crossref] [PubMed]
- Ransohoff DF. Bias as a threat to the validity of cancer molecular-marker research. Nat Rev Cancer 2005;5:142-9. [Crossref] [PubMed]
- Sauerbrei W, Taube SE, McShane LM, et al. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J Natl Cancer Inst 2018;110:803-11. [Crossref] [PubMed]
- Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med 2000;19:453-73. [Crossref] [PubMed]
- Tan MH, Li H, Choong CV, et al. The Karakiewicz nomogram is the most useful clinical predictor for survival outcomes in patients with localized renal cell carcinoma. Cancer 2011;117:5314-24. [Crossref] [PubMed]
- Kattan MW, Hess KR, Amin MB, et al. American Joint Committee on Cancer acceptance criteria for inclusion of risk models for individualized prognosis in the practice of precision medicine. CA Cancer J Clin 2016;66:370-4. [Crossref] [PubMed]


