
Intermittent hypoxia enhances the tumor programmed death ligand 1 expression in a mouse model of sleep apnea
Introduction
The programmed death ligand 1 (PD-L1), as a transmembrane protein of 40 kDa, is expressed on various cells, including cancer cell (1). The PD-1/PD-L1 pathway has been considered significant role in tumor evasion from anti-tumor immune response. Tumors can inhibit the activation of T cells, and induce tumor immune escape via overexpressing PD-L1 ligands on tumor cells surface and binding PD-1 receptor on immune cells (T, B, and NK cells) (2). The overexpression of PD-L1 on lung cancer is associated with poor prognosis (3). Furthermore, anti-PD-1 antibodies, such as nivolumab and pembrolizumab, are accepted as the novel immunotherapeutic choice in cancer (4).
Obstructive sleep apnea (OSA) is associated with cancer incidence and mortality (5,6). As a novel hallmark of OSA, intermittent hypoxia (IH) can stimulate the progress of tumor growth, invasion, and metastasis (7-10). Previous studies have implicated that hypoxia can result in immune suppression, cell proliferation in cancer (11). A study from Noman et al. (12) shows that hypoxic environment can upregulate the expression of PD-L1 on myeloid-derived suppressor cells via hypoxia-inducible factor 1α (HIF-1α).
Most of the prior studies addressed the sustain hypoxia in the tumor microenvironment. A clinical study from Spain found that PD-L1 on monocytes and PD-1 on CD8+ T-cells were increased in patients with OSA as a severity-dependent manner (13). Recently, the same study group also showed that soluble PD-L1 levels increased in melanoma patients combined with severe OSA (14). Few authors studied the influence of IH on the expression of PD-L1 in an experimental study. The present study aims to determine the expression of PD-L1 on tumor tissue in a mouse model of the IH.
Methods
This study was approved by the Ethics Committee of Zhongshan Hospital, Xiamen University (approved number: 2017-015), and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (15).
Animal and subgroup
Twenty-four 7-week-old male C57BL/6 mice were purchased from Chinese Academy of Science Laboratory Animals Center in Shanghai, China and were randomly assigned to control (normoxia) (CTL) group and IH group (n=12 in each group). Excepted for the IH period, mice in the two groups were all housed in standard cages with 12:12-h light-dark cycle and fed freely with food and water. Body weight was measured every week.
Lewis lung carcinoma (LLC) cells culture and tumor induction
LLC cells (obtained from CoBioer Biosciences Co., Ltd. Shanghai, China) were maintained at high glucose DMEM and supplemented with 10% fetal bovine serum (GIBCO, USA). The LLC cells were subcutaneously injected (1×106 LLC in 100 µL PBS of each mouse) into the right flank of mice after 1 week of the IH exposure. The tumor could be palpable and grew rapidly after 7 days of the LLC injection, so the tumor length (L) and width (W) was measured with an electronic caliper (V = W2 × L/2) every 5 days for the tumor volume calculation.
IH exposure
Mice in the IH group were subjected to the IH environment. Exposure was performed according to our previous study (16), with some modification. Mice in the IH group were caged in a self-made plexiglass chamber with one-way valves. The IH cycle consisted of the 50 s of nitrogen, resting for 10 s, oxygen for 10 s, and compressed air for 50 s. Each IH cycle was 120 s, and the nadir chamber oxygen concentration was 6%±1%. The experimental period of IH exposure was from 08:00 AM to 04:00 PM daily during light periods for 5 consecutive weeks (16,17). All mice had free to access water and food during the experimentation period.
Tissue preparation
After 5 weeks of the IH exposure, mice were euthanized and exsanguinated by cardiac puncture for plasma yield. Tumors were excised, weighted, cut in half. One piece of the tumor was frozen in liquid nitrogen and stored at −80 °C for further analysis, and the other was fixed in buffered 10% formalin for histological examination. All the procedures mentioned below were done in all 12 mice of each group.
Western blotting
Tumor tissues were homogenized with RIPA buffer (Beyotime, Beijing, China) in a glass homogenizer, and the supernatants were extracted after by centrifuging. The total protein concentrations in the supernatants were detected by using bicinchoninic acid protein assay (Beyotime, Beijing, China). Boiled proteins (40 µg/lane) were subjected to 10% sodium dodecyl sulfate-PAGE and transferred into polyvinylidene fluoride (PVDF) membranes (Millipore, Boston, MA, USA). After blocked with 5% (w/v) non-fat milk for at least 1 hour at room temperature, the membrane was incubated with the following antibodies at 4 °C overnight: mouse anti-HIF-1α monoclonal antibody (1:250, Novus Biologicals, Littleton, CO), mouse anti-PD-L1 (1:1,000, eBioscienceTM), and mouse anti-β-actin (1:5,000, Santa Cruz Biotechnology, USA). After a rinse with Tris-buffered saline + Tween-20 for 3 times, the membranes were incubated with goat anti-mouse IgG-HRP at 37 °C for 1 hour, and they were developed and exposed using an enhanced chemiluminescence kit (ClarityTM Western ECL Substrate, Bio-Rad). The band densities on the membranes were estimated using the Image J analysis software (National Institutes of Health, Bethesda, MD, USA).
Hematoxylin-eosin (HE) staining and immunohistochemistry (IHC)
The tumor tissue in the formalin was embedded in paraffin, sectioned into 5 µm sections which were stained with HE. IHC analysis of PD-L1 was performed using a standard technique according to the manufactory instruction. Briefly, paraffin-embedded specimens were cut into 5 µm sections and baked at 65 °C for 30 minutes. After deparaffinization, sections were rehydrated through a series of graded ethanol, and then slides were blocked with 10% goat serum (Solarbio, Beijing, China) for 15 min. Endogenous peroxidase activity was blocked by using 3.0% hydrogen peroxide for 10 min prior to being incubated with the primary antibody: mouse anti-PD-L1 (1:200, eBioscienceTM) at 4 °C overnight. The second goat antibody to mouse IgG conjugated with biotin and HRP-streptavidin were used. For antigen visualization, we used avidin-3, 3-diaminobenzidine (DAB) staining kit (Beijing Zhongshan Biotechnology, Beijing, China) to detect immune complexes, and counterstained with hematoxylin. Five random fields were chosen per section and analyzed under 400× magnification by using a Nikon i80 microscope. Integrated optical density (IOD) of each field was measured and analyzed using Image-Pro Plus (version 6.0; Medica Cybernetics, USA).
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was performed for data analysis. All values are presented as mean ± standard deviation. The values between the two groups were compared by independent sample t-test at different timelines. The correlations between tumor weight, tumor volume, and HIF-1α and the expression of PD-L1 was analyzed with Pearson correlation in both groups. Differences were considered significant if the P value less than 0.05.
Results
Body weight, tumor weight, and tumor volume
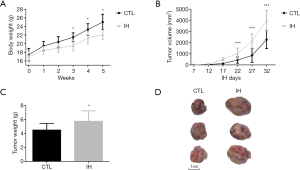
No mouse died after the 5-week experiment. Mice exposed to the IH condition gained less body weight. Both tumor volume and weight were higher in the IH group than those of the CTL group (Figure 1).
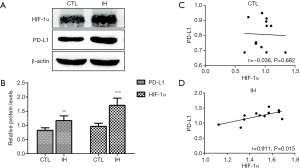
Expression and correlation between HIF-1α and PD-L1
Western blotting results illustrate that mice in the IH group had increased HIF-1α and PD-L1 protein levels than those of the CTL group. The expression of HIF-1α was positively associated with PD-L1 expression in IH exposing mice (Figure 2).
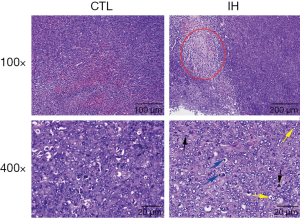
HE staining
From Figure 3, we can find that the phenomena of tumor necrosis, nuclear division, and nuclear deep dyeing were more frequently observed in mice exposed to the IH.
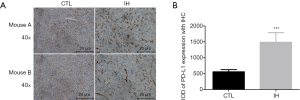
IHC results of PD-L1 expression in tumor tissue
IHC results show that compared with those under the normoxia condition, mice under the IH condition presented elevated PD-L1 expression levels (Figure 4).
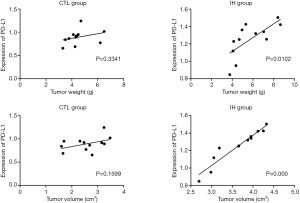
Correlation between tumor weight, tumor volume, and PD-L1 expression
Figure 5 illustrated that tumor weight and volume are correlated with the expression of PD-L1 in the IH group (all P<0.05). Dissimilar findings were observed in the CTL group.
Discussion
This study established the IH and tumor models in one mouse, and the results illustrated that compared to mice under normoxia condition, those under the IH condition had high PD-L1 expression in tumor tissue. Both tumor weight and volume were associated with the expression of PD-L1 in the IH group.
OSA is a common medical condition among middle-aged people. The recurrent collapse of the upper airway during sleep leads to chronic IH and sleep fragmentation. Robust data confirm the relationship between OSA and cardiovascular disease, hypertension, and metabolic abnormalities (18). Recently, accumulating evidence confirmed the association between OSA and cancer incidence and mortality (6,19-21). Zhang et al. (22) elicited that OSA may increase the risk for primary central nervous system cancer development. The Wisconsin Sleep Cohort Study (19) demonstrated that after adjustment for confounding factors, sleep-disordered breathing (SDB) was associated with cancer mortality in a dose-response fashion, and the relative hazard in severe SDB patients was 4.8 (95% confidence interval: 1.7–13.2). Furthermore, IH can promote tumor growth, proliferation, invasion, and metastasis (7-9) in animal studies. Our study was consistent with previous studies, finding that mice under IH condition had higher tumor weight and volume than those of the normoxia condition. The underlying molecular mechanism is yet uncertain. A study from Almendros et al. (10) supported that IH-induced alterations in tumor-associated macrophages play an important role in OSA enhancing the cancer progress. Akbarpour et al. (23) found decreasing CD8+ T cells and impaired cytolytic function, increasing CD4+, CD44+, and CD133+ expressing cancer stem cells in tumor-bearing mice under the IH exposure. The author considered that the abovementioned changes in tumor microenvironment under the IH condition may result in reduced immune-surveillance and adverse tumor outcomes (23). The IH aggravates tumor adverse outcome, however, few interventional study was conducted to evaluate certain treatment on cancer in OSA patients.
Immune evasion which enables cancer cells to escape the attack from the immune system is a considerable hallmark of cancer. Several immune inhibitory signaling molecules expressed on cancer cells leads to immune cell dysfunction and apoptosis. One significant inhibitory molecule is PD-L1. Combining with PD-1 expressed on immune cells, PD-L1 can protect cancer cell against the attack of immune cells. Namely, the expression of PD-1/PD-L1 can suppress anti-cancer immunity. Previous studies demonstrated that the high expression of PD-L1 is correlated with poor prognosis in various types of cancers (3,24,25). Cancer treatment with anti-PD-L1 and anti-PD-1 antibodies can improve the overall survival, and progression-free survival (4). Previous clinical trials showed a significant anti-cancer activity of both anti-PD-L1 and anti-PD-1 antibodies (26,27). The response rate of 10–20% was found in patients with non-small-cell lung cancer. Further analysis of cancer specimens revealed that there was a positive correlation between PD-L1 expression and response rate (26,27). Hypoxia in the tumor microenvironment leads to the increased HIF-1α levels which are considerably correlated with high PD-L1 expression and damage the T-cell function and contributing to a poor prognosis of cancer patients (28,29). Different from sustained hypoxia, IH which existing in OSA patients is not widely studied in cancer patients (18). Whether the tumor has aberrant PD-L1 expression after the IH exposure is unclear. The present study determined the high tumor PD-L1 expression in the IH-induced mice. The results elicited that compared to the normoxia condition, elevated tumor PD-L1 expression was detected in mice under the IH condition. We further found that tumor weight, tumor volume, and HIF-1α expression were positively correlated with the PD-L1 expression. Our findings were consistent with the previous study by Cubillos-Zapata and co-authors (13). They studied the PD-L1 on monocytes and PD-1 on CD8+ T-cells expressions on immune cells from OSA patients. Increased PD-1 and PD-L1 expressions were detected in OSA patient, and the increased levels were in a severity-dependent manner. Further analysis in their study found that apnea-hypopnea index and oxygen desaturation index, two polysomnographic variables, were tightly associated with the PD-L1 expression (13). More recently, the same authors with a multicenter observational study (14) showed that soluble PD-L1 expression was significantly high in melanoma patients with severe OSA, they speculated that soluble PD-L1 might serve as a potential biomarker of melanoma aggressiveness and invasiveness in these patients. From the studies of our group and Cubillos-Zapata and co-authors (13,14), we concluded that the high PD-L1 expression attributes to the IH mimicking OSA.
This study has its own limitations. Firstly, we only observed the phenomenon that tumor weight and volume are associated with the PD-L1 expression under the IH condition, the underlying mechanism between IH and PD-L1 was not analyzed. More attention should be paid in the immune-editing process (such as myeloid-derived suppressor cells, TAMs, NK cells, Treg, etc.) for an explanation of the correlation between PD-L1 expression and other changes in the immunomodulatory mechanisms induced by the IH. Secondly, we only compared the PD-L1 expression under the normoxia and IH conditions, anti-PD-1/PD-L1 antibodies treatment was not used to verify the results of this study. Thirdly, the tumor grew rapidly, especially in the IH group (8), the tumor size was relatively big at the end of the experiment, further study should pay more attention to the study period.
Conclusions
This study shows that IH can enhance the tumor PD-L1 expression in mice mimicking OSA. The results can provide preliminary experimental evidence for patients suffering from OSA and cancer. Further experimental and clinical studies are needed to verify the results of our study.
Acknowledgements
Funding: This work was supported by Grant 3502Z20154019 for Fund from Xiamen Science and Technology Bureau, Grant 2018-2-65 for Youth Research Fund from Fujian Provincial Health Bureau, and Grant 2018J01393 for Fund from Natural Science Foundation of Fujian Province, China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Zhongshan Hospital, Xiamen University (approved number: 2017-015), and conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
References
- Butte MJ, Pena-Cruz V, Kim MJ, et al. Interaction of human PD-L1 and B7-1. Mol Immunol 2008;45:3567-72. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 2014;7:567-73. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Gozal D, Ham SA, Mokhlesi B. Sleep Apnea and Cancer: Analysis of a Nationwide Population Sample. Sleep 2016;39:1493-500. [Crossref] [PubMed]
- Martinez-Garcia MA, Campos-Rodriguez F, Duran-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med 2014;15:742-8. [Crossref] [PubMed]
- Almendros I, Montserrat JM, Ramirez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J 2012;39:215-7. [Crossref] [PubMed]
- Almendros I, Montserrat JM, Torres M, et al. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med 2012;13:1254-60. [Crossref] [PubMed]
- Almendros I, Montserrat JM, Torres M, et al. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol 2013;186:303-7. [Crossref] [PubMed]
- Almendros I, Wang Y, Becker L, et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med 2014;189:593-601. [Crossref] [PubMed]
- Barsoum IB, Koti M, Siemens DR, et al. Mechanisms of hypoxia-mediated immune escape in cancer. Cancer Res 2014;74:7185-90. [Crossref] [PubMed]
- Noman MZ, Chouaib S. Targeting hypoxia at the forefront of anticancer immune responses. Oncoimmunology 2015;3:e954463. [Crossref] [PubMed]
- Cubillos-Zapata C, Avendano-Ortiz J, Hernandez-Jimenez E, et al. Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in sleep apnoea. Eur Respir J 2017.50. [PubMed]
- Cubillos-Zapata C, Martínez-García MÁ, Campos-Rodríguez F, et al. Soluble PD-L1 is a potential biomarker of cutaneous melanoma aggressiveness and metastasis in obstructive sleep apnea patients. Eur Respir J 2019;53. [Crossref] [PubMed]
- National Research Concil (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press (US), 2011.
- Zhang XB, Yang YY, Zeng Y, et al. Anti-tumor effect of endostatin in a sleep-apnea mouse model with tumor. Clin Transl Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Almendros I, Gileles-Hillel A, Khalyfa A, et al. Adipose tissue macrophage polarization by intermittent hypoxia in a mouse model of OSA: effect of tumor microenvironment. Cancer Lett 2015;361:233-9. [Crossref] [PubMed]
- Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014;383:736-47. [Crossref] [PubMed]
- Nieto FJ, Peppard PE, Young T, et al. Sleep-disordered Breathing and Cancer Mortality Results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2012;186:190-4. [Crossref] [PubMed]
- Marshall NS, Wong KK, Cullen SR, et al. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the busselton health study cohort. J Clin Sleep Med 2014;10:355-62. [PubMed]
- Kendzerska T, Leung RS, Hawker G, et al. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ 2014;186:985-92. [Crossref] [PubMed]
- Zhang XB, Jiang XT, Lin QC, et al. Effect of continuous positive airway pressure on serum cystatin C among obstructive sleep apnea syndrome patients. Int Urol Nephrol 2014;46:1997-2002. [Crossref] [PubMed]
- Akbarpour M, Khalyfa A, Qiao Z, et al. Altered CD8+ T-Cell Lymphocyte Function and TC1 Cell Stemness Contribute to Enhanced Malignant Tumor Properties in Murine Models of Sleep Apnea. Sleep 2017.40. [PubMed]
- Chen J, Jiang CC, Jin L, et al. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol 2016;27:409-16. [Crossref] [PubMed]
- Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011;28:682-8. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Barsoum IB, Smallwood CA, Siemens DR, et al. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 2014;74:665-74. [Crossref] [PubMed]
- Shehade H, Oldenhove G, Moser M. Hypoxia in the intestine or solid tumors: a beneficial or deleterious alarm signal? Eur J Immunol 2014;44:2550-7. [Crossref] [PubMed]


