Clarification of the resection line non-intubated segmentectomy using indocyanine green
Background
Segmentectomy
A pulmonary segmentectomy is an anatomical resection which is used in curative surgery of pulmonary pathologies. The pioneer of this method is Edward Churchill. In the beginning, segmentectomy was indicated in the treatment of inflammatory pulmonary diseases (1). The first surgeon using segmentectomy for lung cancer treatment was Richard Overholt (2). The gold standard of primary lung cancer treatment was for decades lobectomy (3,4). For patients with limited ventilatory function, the extent of pulmonary resection is limited in a way that a lobectomy is contraindicated. A wedge resection is associated with a higher occurrence of relapses (5). These facts lead to research into the biological justification of sub-lobar anatomical pulmonary resections (6-8). This occurred in connection with the expansion of early diagnoses of lung cancers which arose in the 1980s and 1990s thanks to Michael Melamed, Claudia Henschke and their coworkers (9,10). This leads to a number of surgeons replacing lobectomy in the treatment of early pulmonary cancer with segmentectomy. At the end of same decade, this trend sparked a sharp exchange of opinions at a conference in Italian Varese which was organized by Lorenzo Dominioni and Gary Strauss (11). Modernists offered impulses for fruitful muses (12). Conservatives observed the purity of the concept via tough sorties (5). While classicists had technical details already polished for a long time (1,6,13).
The indication of pulmonary segmentectomy for cancer up to a diameter of 20 mm came rose victoriously from the aforementioned exchange of opinions. Credit should be given to Japanese surgeons led by Shigefumi Fujimura et al. (8), who were supported especially by the authority of Penfield Faber from Chicago (6,13). Surgical practice in the following decade has shown that this decision was correct (14-17) and we have used this method of surgical oncology since 1999 (18).
Non-intubated thoracic surgery (NITS)
The basis of today’s physiological thoracic operation is non-intubated anatomical lung resection with regional anesthesia of the chest wall and intercostal nerves (lidocaine, bupivacaine), a vagus nerve block in the pulmonary hilum (bupivacaine) and pharmacological sedation [target controlled infusion (TCI)] with propofol (19). There are both proponents and opponents of this method, as of any other. In addition to its advantages, NITS also has its limitations: BMI <27, significant weighty movement of the thoracic wall, lung and mediastinum associated with artificial pneumothorax, extensive pleural adhesions and potential allergic reactions to the medication used. When we become more familiar with NITS, we find that it is notably effective in indicated cases. Our experience shows a difference in the reachability of the Asiatic and Caucasian population. Their causation is possibly multifactorial, and are worthy of focused interest. NITS combines the advantages of minimally-invasive video-assisted thoracic surgery (VATS) and spontaneous breathing without intubation. NITS eliminates complications related to selective double-lumen intubation, artificial breathing, and general anesthesia. A look into the literature shows that NITS is not an achievement of the 21st Century, but it has developed concurrently with intubated surgery using artificial breathing (20-22).
The specification of the intersegmental plane
A classic open pulmonary segmentectomy with elegant digitoclastic technique is able to separate intersegmental planes of the pulmonary parenchyma exactly. Deciphering this process for VATS was performed by Koichi Yoshikawa with colleagues (14) in the concept of extended segmentectomy; and more recently, Noriyuki Misaki using indocyanine green (ICG) (23,24).
Gaetano Rocco with colleagues proposed blocking the entrance into the segmental bronchus using a Forgarty catheter via bronchoscopy (25).
Anatomical, physiological and biophysical prerequisites
The pulmonary segment is perfused via a segmental branch of the pulmonary artery and ventilated via the segmental bronchus. Systemic circulation brings a slight amount of arterial blood into the bronchial artery. The blocking of blood inflow to the pulmonary segment by cutting off the segmental pulmonary artery can be used to visualize the resection line. Soon after the application of ICG into the peripheral vein, ICG appears in the pulmonary circulation. Electromagnetic near infrared (NIR) wave motion stimulates its fluorescence. ICG flows into the perfused segments, so they will appear fluorescent and dyed green. ICG will not flow to non-perfused segments, so they will not appear green. This method delimits them in relation to surrounding segments.
ICG
ICG is a cyanine dye characterized by absorption of the near infrared spectrum. The absorption peak is 805 nm. The exact range of its fluorescence depends on the chemical characteristics of its surroundings and the physical conditions of ICG molecules, such as temperature or concentration of the solution. The spectra of fluorescence also slightly changes, because exact wavelengths described in the literature are slightly different according to spectrums of excited light wave motion and according to the filters used to detect emitted light. ICG clinical use has the following advantages which caused its quick acceptance: maximal absorption around 800 nm, its limitation to the vascular compartment through their bond to plasma proteins, low toxicity and quick expulsion—almost exclusively via the liver to bile (9). To establish the amount of intravenous dosage in studies regarding liver function, 0.5 mg/kg is calculated. The total dosage of ICG for the heart’s output and volume of blood should be under 2 mg/kg. No significant toxic effects were observed up to a dose of 5 mg/kg (26). ICG is the only fluorescence dye approved by the Food and Drug Administration (FDA) for use in the biomedicine.
In accordance with the aforementioned information, we considered clarifying the intersegmental plane of the pulmonary segment using ICG after its stimulation by electromagnetic wave motion from the near infrared part of spectrum during the NITS procedure. We decided to test this.
Case presentation
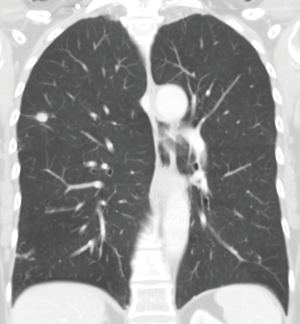
An ICG-navigated segmentectomy was performed in a 60-year-old woman with a solitary lesion 13 mm in diameter in the posterior segment of the upper right pulmonary lobe (Figure 1, CT scan). The operation was performed using a non-intubated thoracic surgical procedure. The spontaneously breathing patient was set on the left decubital position and sedated using propofol. Pain and the cough reflex were inhibited by an intercostal nerve block, a block of the vagus nerve in the pulmonary hilum, and topical anesthesia of the visceral pleura with bupivacaine. Using the combination of blunt and sharp preparation the segmental artery, and bronchus were found and secured. Next, the segmental artery was cut using a Medtronic EndoGIA 45 mm vascular stapler. After cutting the segmental pulmonary artery, ICG was applied in concordance with the protocol of the ethical committee and the free and informed consent of the patient. We used ICG, specifically the commercially-available ICG pulsion from Sigma Aldrich. We applied 2 mL of the solution, which contained 5 mg of ICG (0.1 mg/kg).
The lower concentration of ICG was chosen due to the experience from gastrointestinal tract surgery regarding the non-linear correlation between the concentration of ICG and fluorescence quantum yield (26). The essential factor when using this indicator was the penetration of the NIR wave motion at least several few millimeters into depth of the pulmonary tissue (27,28). The ICG was excited using laser radiation from endoscopic Novadaq Pinpoint Endoscopic Fluorescence Imaging display system, system wave length 805 nm, 20 pulses/sec, output 2 mW, and 75°±5° divergence of the ray beam. The emitted ICG fluorescence was detected by a CMOS camera and visualized on a screen via video convertor of the same endoscopic system. After a few seconds of intravenous application of the ICG, we noticed the first coloration of the pulmonary parenchyma. Despite the information in the literature we did not detect clear borders of saturation loss of the pulmonary segment which was meant for resection. The segmental bronchus was at this time intact. The surgery continued with a lymphadenectomy. After half an hour, once complete washout of the ICG from the pulmonary tissue was obvious, we severed the segmental bronchus with a stapler using a green cartridge.
Afterwards, we repeated the application of ICG into the peripheral vein. Two milliliters of the solution were applied again. The segment with the interrupted segmental branch of the pulmonary artery and severed segmental bronchus did not change colour, but again a clear border for marking the resection line was not noticeable. We separated the posterior segment at the assumed borderline from the surrounding parenchyma using staplers with blue cartridge and resection was extracted via a utility incision in the chest wall. A histopathology examination did not confirm the assumed neoplastic etiology of the lesion. Post-inflammatory changes were documented. The postoperative course was without issue.
We were surprised to find that the visual demarcation of the segment borders using ICG fluorescence was not very sharp in any interval of the observed progression time (Figures 2,3).
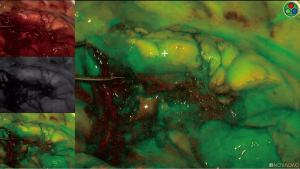
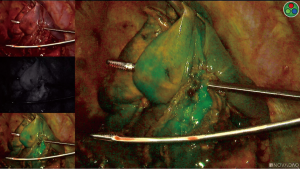
This posed many questions, and serve as an invitation to future tests:
- Biological: the influence of general and local regulation of perfusion and ventilation by partial pneumothorax; presence/involvement of system circulation via bronchial artery.
- Surgical: reflex response of pulmonary tissue and vessels to mechanical manipulation under sedation.
- Pathological: post-inflammatory field with bronchiectasis.
- Pharmacological: vagus nerve block, intercostal block, topic local anesthesia of visceral pleura, drug interaction with ICG, and propofol administration.
- Technological: impact of infrared electromagnetic wave motion on the lung in the location of partial pneumothorax; high flow rate nose oxygen application.
- Physical-chemical: fluorescence quantum yield of ICG, ICG concentration, aggregation of ICG in the solution, pH of surroundings, the dispersion of emitted light in the tissue.
- Technical: the sequence of closing segmental pulmonary artery and segmental bronchus.
- Particular: only one case study.
Tommaso Claudio Mineo and Federico Tacconi showed encouraging results with NITS by paying attention to more aspects needed for study (29).
Video-assisted pulmonary segmentectomy is not an easy or quick procedure. Specifying borderlines of the segment both surgically and comprehensibly and at the same time precisely oncologically is a fundamental question. The first use of ICG in pulmonary segmentectomy was published in 2009 by Noriyuki Misaki et al. (23). Japanese colleagues are pioneers in using ICG in pulmonary surgery. From 2010 to 2014, Kasai et al. conducted several studies using ICG in the medical treatment of patients with pulmonary tumors.
Sébastien Guigard from Geneva, with colleagues, published a study in 2017 on the use of ICG in 22 patients who had undergone VATS (30) and showed that intravenous application is a reliable tool for pinpointing the intersegmental plane. They describe the application of 12.5 mg of ICG, which is a 2.5 times higher dosage than was recommended for a standard preoperative angiography in colorectal surgery (31). The authors were able to determine the intersegmental plane for every patient. Yoshitaka Kasai and colleagues used 0.5 mg/kg of ICG and were able to visualize resection edges in 19 out of 20 patients (32), and Shintaro Tarumi used a dosage of 3 mg/kg of ICG with very good visualization of a resection line in 11 out of 13 patients (33).
We applied 5 mg of ICG (i.e., 0.1 mg/kg) to our patient after cutting the segmental artery. We repeated the application after cutting the segmental bronchus. The low dosage could be the reason our procedure did not bear the intended results. A higher concentration of ICG in the circulation would lead to more noticeable dying of the perfused segments. In the situation in which peripheral vascular communication of centrally perfused segments and the segment without central perfusion are established and open, one cannot logically expect that even with a higher or high dosage of coloring agents would we be able to delimit the resection line. One cannot conclude, based on material which we have, if this vessel-bridging is caused by an inflammatory process in the parenchyma, as mentioned by the histopathologist, or if it is a physiological or other consequence of partial pneumothorax (which is an integral part of NITS).
In addition, we also noticed that after cutting the segmental artery and segmental bronchus, a small green area in the resection fluoresced. The presence of small vascular connections which went beyond the intersegmental planes as an anatomical variation of thoracic surgical experience confirms—like the liver (34)—which we must anticipate, but at the same time we cannot predict. Adapting to an unusual reality is part of our profession.
Conclusions
ICG fluorescence stimulated by infrared electromagnetic wave motion did not make it easier to pinpoint the intersegmental plane during NITS in our patient. Further study is needed.
Acknowledgements
Funding: Support of grants from MZČR-RVO (FNBr, 65269705) and MUNI/A/1011/2017.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Churchill ED, Sweet RH, Soutter L, et al. Surgical Management of Carcinomas of the Lung; A Study of the Cases Treated at the Massachusetts General Hospital from 1930 to 1950. J Thorac Surg 1950;20:349. [PubMed]
- Overholt RH. Surgery for pulmonary cancer. Dis Chest 1956;29:595-604. [Crossref] [PubMed]
- Uglov FG. Pulmonary Resection. In: Russian Rezekcia legkich. 2nd Edn. Medgiz, 1954.
- Ramsey HE, Cahan WG, Beattie EJ. The importance of radical lobectomy in lung cancer. J Thorac Cardiovasc Surg 1969;58:225-30. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-years survival and patterns of intrathoracic recurrence J Thorac Cardiovasc Surg 1994;107:1087-93. [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 1997;114:347-53. [Crossref] [PubMed]
- Fujimura S, Endo C, Saito Y, et al. Radical segmentectomy for roentgenographically occult bronchogenic squamous cell cancer Ann Thorac Surg 1995;60:615-23.
- Melamed MR, Flehinger BJ, Zaman MB. Impact of early detection on the clinical course of lung cancer Surg Clinof North Am 1987;67:909-24.
- Henschke CI, Miettinen OS, Yankelevitz DF, et al. Radiographic screening for cancer. Proposed paradigm for requisite research. Clin Imaging 1994;18:16-20. [Crossref] [PubMed]
- Dominioni L, Strauss GM. Consensus statement. International Conference on Prevention and Early Diagnosis of Lung Cancer. Cancer 2000;89:2329-30. [Crossref]
- Strauss GM, Gleason RE, Sugarbaker DJ. Screening for Lung Cancer: another look; a different view. Chest 1997;111:754-68. [Crossref] [PubMed]
- Jensik RJ, Faber LP, Milloj FJ, et al. Segmental resection for lung cancer: a fifteen years experience. J Thorac Cardiovasc Surg 1973;66:563-72. [PubMed]
- Yoshikawa K, Tsubota N, Kodama K, et al. Prospective study of extended segmentectomy for small lung tumors: The Final Report. Ann Thorac Surg 2002;73:1055-8. [Crossref] [PubMed]
- Nonaka M, Kadokura M, Yamamoto S, et al. Tumor dimension and prognosis in surgically treated lung cancer: for intentional limited resection. Am J Clin Oncol 2003;26:499-503. [Crossref] [PubMed]
- Okada M. Radical sublobar resection for lung cancer Gen Thorac Cardiovasc Surg 2008;56:151-7. [Crossref] [PubMed]
- Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer Eur Respir J 2009;33:426-35. [Crossref] [PubMed]
- Horváth T. "Pulmonary segmentectomy for tumour" in Czech Plicní segmentektomie pro nádor. Rozhl Chir 2009;88:238-47. [PubMed]
- Struys MM, De Smet T, Glen I, et al. The History of Target-Controlled Infusion. Anesthesia Analgesia 2016;122:56-69. [Crossref] [PubMed]
- Buckingham WW, Beatty J, Brasher CA, et al. The Technique of Administering Epidural Anesthesia in Thoracic Surgery. Dis Chest 1950;17:561-8. [Crossref] [PubMed]
- Ossipov BK. Local anesthesia in thoracic surgery: 20 years experience with 3265 cases. Anesthesia Analgesia 1960;39:327-32. [Crossref] [PubMed]
- Katlic MR, Facktor MA. Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases. Ann Thorac Surg 2010;90:240-5. [Crossref] [PubMed]
- Misaki N, Chang SS, Gotoh M, et al. A novel method for determining advanced lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. [Crossref] [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Rocco G, Romano V, Accardo R, et al. Awake-single access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting Ann Thorac Surg 2010;89:1625-7. [Crossref] [PubMed]
- Landsman ML, Kwant G, Mook GA, et al. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J Appl Physiol 1976;40:575-83. [Crossref] [PubMed]
- Alander JT, Kaartinen I, Laakso A, et al. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int J Biomed Imaging 2012;2012:940585. [Crossref] [PubMed]
- Boni L, David G, Dionigi G, et al. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc 2016;30:2736-42. [Crossref] [PubMed]
- Mineo TC, Tacconi F. From “awake” to “monitored anesthesia care” thoracic surgery: A 15 year evolution. Thorac Cancer 2014;5:1-13. [Crossref] [PubMed]
- Guigard S, Triponez F, Bédat B, et al. Usefulness of near-infrared angiography for identifying the intersegmental plane and vascular supply during video-assisted thoracoscopic segmentectomy. Interact Cardiovasc Thorac Surg 2017;25:703-9. [Crossref] [PubMed]
- Foppa C, Denoya PI, Tarta C. Indocyanine green fluorescent dye during bowel surgery: Are the blood supply “guessing days” over? Tech Coloproctol 2014;18:753-8. [Crossref] [PubMed]
- Kasai Y, Tarumi S, Chang SS, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2- and 1-wavelength methods. Eur J Cardiothorac Surg 2013;44:1103-7. [Crossref] [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
- Clavien PA, Lillemoe KD. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. Ann Surg 2016;263:835-6. [Crossref] [PubMed]