
Pulmonary nodular lymphoid hyperplasia: a rare benign disease with malignant mask
Introduction
Pulmonary nodular lymphoid hyperplasia (PNLH), initially termed as pseudolymphoma (1), is a rare benign disease. It was firstly introduced by Kradin and Mark (2) to describe one or more nodules or localized lung infiltrates, which consisted of a reactive lymphoid proliferation. Because of the rarity of PNLH and few reports on its clinical features such as hematologic index or radiological imaging, the preoperative diagnosis of PNLH is difficult even by PET-CT or needle biopsy. Its diagnosis mainly relies on postoperative pathological and immunohistochemical examination (3-6). There are several studies reporting that PNLH could be easily misdiagnosed as lung adenocarcinoma or lymphoma and the surgical resection for patients with adequate pulmonary function is both diagnostic and curative (4,7-9). The prognosis after surgical intervention is good (3), however, most of the patients with such benign disease who have undergone complete lobectomy would suffer substantial loss of normal lung parenchyma.
Compared with lobectomy, sublobar resection procedures such as wedge resection and segmentectomy have been proven to preserve more pulmonary function and decrease the occurrence of perioperative complications (10-12), and have advantages in the enhanced recovery after surgery (ERAS). Therefore, on the premise of equivalent or even better efficacy and outcome, sublobar resection has the priority in treating certain pulmonary diseases, especially benign disease. Although researchers have reported that the efficacy and outcome of segmentectomy or wedge resection for PNLH is considered to be excellent (5,13), there is no comparison of perioperative and long-term outcomes among different surgical approaches with valid evidence.
In this study, we aim to explore the clinical features of PNLH, including individual characteristics, radiological findings and long-term survival and to compare perioperative data of PNLH patients with sublobar resection and those with lobectomy. The primary goal of this study is to summarize the main clinical features of PNLH and to determine the optimal surgical approach for PNLH in terms of intraoperative and postoperative outcomes.
Methods
Patients and surgical procedure
Patients who underwent surgical treatment for PNLH at the First Affiliated Hospital of Zhejiang University, School of Medicine between March 2007 and August 2017 were consecutively included. The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Zhejiang University, School of Medicine (ID of ethics approval: 20181032). The clinical information was collected from hospital electronic medical records system, including demographic characteristics, medical history, preoperative investigations, intraoperative data and postoperative course. PNLH was confirmed through pathological and immunohistochemical examinations in the resected pulmonary specimens. Lesion size was defined as the maximum diameter. Patients with a senior high school education or above were defined as well-educated, others were classified as poorly-educated. Smoking and drinking was defined as historical or current tobacco and alcohol dependence, respectively.
All patients were evaluated thoroughly before the surgery and only those with sufficient cardiopulmonary function were suitable as the candidates for thoracotomy or video-assisted thoracoscopic surgery (VATS). It was worth mentioning that only three patients underwent PET-CT scans before surgery to assess the lesions. There were three reasons. First, 44 patients did not have the opportunity to take the examination because the machine of PET-CT was introduced into our center in late 2014. Secondly, 3 patients were very highly suspected as early stage lung cancer on CT scan and underwent surgery directly without taking PET-CT scan. Finally, the remaining patients refused to take this examination due to the high cost. Wedge resection was adopted with priority if parenchymal resection margins ≥2 cm could be achieved. Segmentectomy was performed if it was difficult to preserve the adequate margin by wedge resection. There were three criteria performing lobectomy. First, the lesion was too extensive to be guaranteed the negative margin after sublobar resection. Secondly, the lesion was located too deep to be removed by sublobar resection. Finally, the lesion was larger than 2 cm and highly suspected as lung cancer.
All patients underwent general anesthesia with single-lung ventilation and were placed in lateral decubitus position. Conventional posterolateral serratus divided thoracotomies were performed in the open procedures, and 3-ports approach was adopted in the thoracoscopic procedures. Generally, bronchi, pulmonary vasculature and parenchyma were resected by the corresponding endoscopic cut stapler. All resected specimens were routinely sent for frozen section examination during operation. Prior closing, the cavity was rinsed by fairly high temperature of normal saline to detect potential air leak and one chest tube was placed in the appropriate position at the end of the procedure. The tube was removed when it was clearly confirmed no air leak and the volume of drainage was less than 200 mL/day. Patients were discharged from the hospital if there was no postoperative complication and no obvious pneumothorax or chest fluid by roentgenograms. Regular reexamination was required for all patients after being discharged from the hospital.
Follow-up
Follow-up data were collected by telephone calls and reviewing the records of reexamination in the outpatient clinic. The last follow-up time was February 2018. The outcomes of interest of the current study included the incidence of disease recurrence, disease-free survival, and overall survival. Recurrence was defined as PNLH relapse within the same or other lobes of the lung confirmed through histological or radiographic methods.
Statistical analysis
All the analysis was conducted by SPSS software (version 19.0, IBM SPSS Inc., United States). The measurement data and numeration data were statistically analyzed with t test and χ2 test respectively. Statistical significance was set at P value <0.05 (All P values presented were 2-sided).
Results
Clinical characteristics
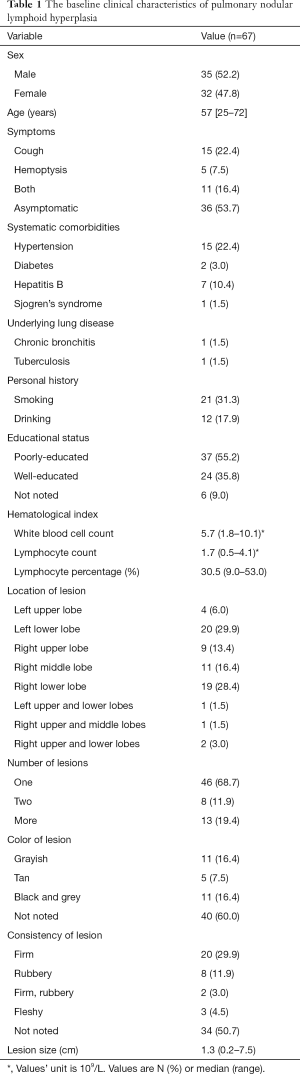
From March 2007 to August 2017, a total of 67 patients with PNLH were diagnosed in our department. The major characteristics were listed in Table 1. There was no significant gender difference among the included patients and the median age was 57 years old, which ranged from 25 to 72 years. More than half of the nodules were detected by routine chest X-rays or computed tomography (CT). There was one patient with Sjogren’s syndrome. In terms of hematological index, lymphocyte count and percentage were normal in most of the patients. Serum tumor makers were normal except a 57-year old male patient with elevated ferritin. Lesions were more likely to be located in the lower lobes of the lung and 4 cases have been found involving two lobes. 46 of 67 patients (68.7%) had solitary lesions, whereas 21 of 67 (31.3%) demonstrated two or more lesions. Most of lesions were grayish, tan, black and grey nodules or masses with a firm, rubbery, or fleshy consistency. The lesions varied from 0.2 to 7.5 cm in the greatest dimension with a mean maximum diameter of 1.8 cm. Most of the lesions were less than 3 cm in the greatest dimension.
Full table
Imaging findings
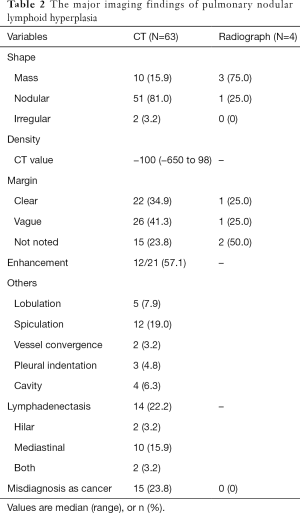
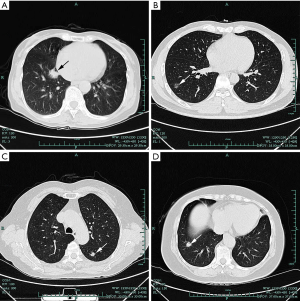
All patients received imaging examination before the surgery. CT scans were performed in 63 patients, 21 of whom underwent enhanced CT scanning while chest radiographs were performed in 4 patients. The major imaging findings of lesions were summarized in Table 2. Most lesions presented as subsolid or solid nodules and part of them possessed the characteristics of pulmonary adenocarcinoma, such as lobulation, speculation, vessel convergence, pleural indentation, enhancement and lymphadenectasis. Thus, 24% of the patients who underwent CT scans were considered of having lung cancer by the radiologists (Figure 1). Additionally, there were three patients who underwent PET-CT scan examinations. Two cases showed that FDG uptake elevated slightly with standardized uptake values (SUV) of 0.99 and 0.91 respectively, while the remaining one was negative.
Full table
Surgical procedure and perioperative data
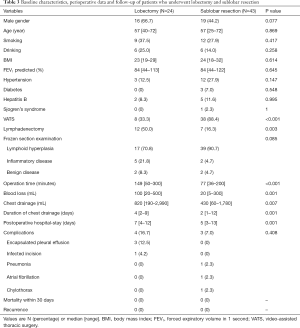
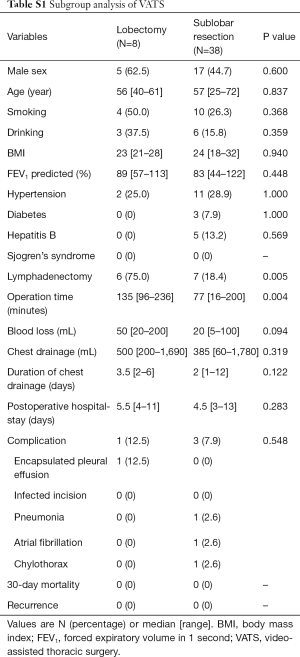
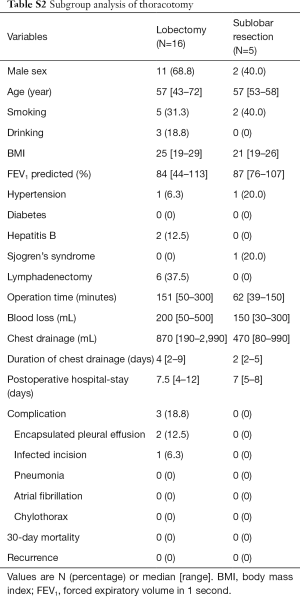
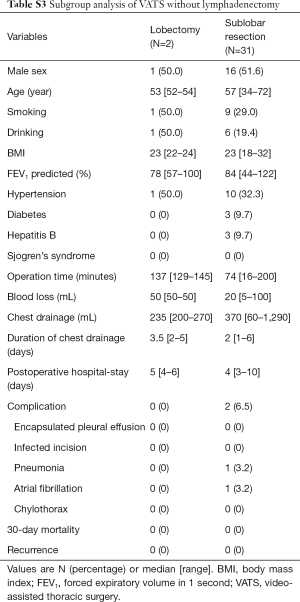
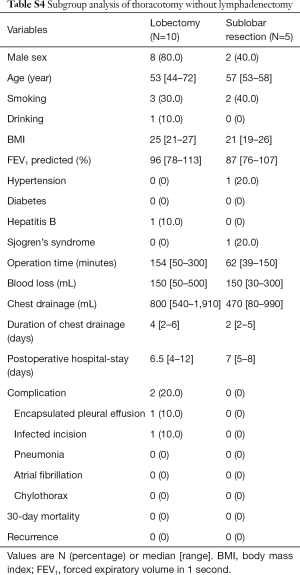
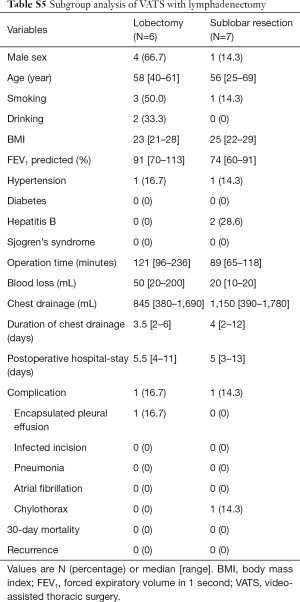
Twenty-four of 67 patients underwent lobectomy, including one case transformed from video-assisted thoracic surgery (VATS) to thoracotomy due to severe pleural adhesion, and 43 patients underwent sublobar resection, including one patient who underwent segmentectomy and 42 patients who underwent wedge resection. The baseline demographic characteristics and perioperative information of the two groups were shown in Table 3. The lesions were diagnosed by intraoperative frozen section examination as lymphoid hyperplasia, inflammatory disease and benign disease in 17, 5, 2 patients in the lobectomy group, and 39, 2, 2 patients in the sublobar resection group, respectively, with no statistical difference between two groups. In comparison with the lobectomy group, the sublobar resection group tended to take shorter operative time (149 vs. 77 min; P<0.001) and less amount of blood lost (100 vs. 20 mL; P=0.001) intraoperatively. Additionally, sublobar resection also had advantages on the volume (820 vs. 430 mL; P=0.007) and duration of chest drainage (4 vs. 2 days; P=0.001), as well as the postoperative hospital stay (7 vs. 5 days; P=0.001). There was a 60-year old female patient in sublobar resection group who underwent lymphadenectomy and later developed chylothorax postoperatively. After the conservative treatment such as sufficient drainage, she was discharged from hospital on postoperative day 13. In general, there was no statistical differences in perioperative complications. No dead event occurred during 30 days after the operation. All the pathological results of resected lymph nodes were reactive hyperplasia or inflammatory change. The rates of VATS and lymphadenectomy between two groups had statistical differences, and further subgroup analysis was conducted to investigate the potential heterogeneity among different groups, as shown in the Tables S1-S5.
Full table
Full table
Full table
Full table
Full table
Full table
Follow-up
Follow-up information was successfully obtained from 50 of 67 patients with median follow-up time of 43 months (range: 6 to 129 months). All patients were alive except one who died of accident after lobectomy, and there was no documented recurrence being observed in any cases during the follow-up period.
Discussion
The understanding of PNLH was limited. So far, the study on PNLH with the largest sample size was reported by Bois et al. (14), which enrolled 26 patients. Bois et al. primarily focused on the differences between PNLH and immunoglobulin (Ig) G4–related lung disease (IgG4-RLD) in terms of serum IgG4 level and IgG4+/IgG+ ratio, yet did not specifically discuss the clinical features and treatment of PNLH in detail. In 2000, Abbondanzo et al. presented 14 cases, indicating that PNLH showed several constant histological features which included exuberant, reactive germinal centers, interfollicular mature plasma cells (often in sheets) and small reactive lymphocytes, and varying amounts of interfollicular fibrosis (3), but the imaging manifestations and the choice of surgical approaches were still poorly understood.
In the current study, we collected more patients with PNLH than all the previous studies. We explored the major clinical characteristics of patients and provided the single-center experience on the treatment of PNLH. The patients’ characteristics were similar to those described by previous studies (3,15). No gender difference was found in PNLH patients and most of them were middle-aged and elderly. There was one patient with Sjogren’s syndrome in our study and it was also reported that PNLH had the potential to share characteristics with such autoimmune diseases as Sjögren’s syndrome (16-18). However, the evidence was inadequate to prove the correlation between PNLH and autoimmune diseases.
The diagnosis of PNLH is difficult preoperatively and mainly relies on pathological and even immunohistochemical examinations postoperatively. In our study, more than half of the patients were asymptomatic and few presented with nonspecific symptoms such as cough and hemoptysis. The lesions were usually detected initially through chest radiographs or CT scans. However, the imaging findings of PNLH, including PET-CT, were very similar to those of malignant tumors (7,8,19), and even the aspiration biopsy might also be unhelpful (4,8,13). Thus, PNLH is a benign disease with malignant mask. Based on our single-center experience, the radiological manifestations of PNLH commonly presented with a solitary solid or subsolid nodule and occasionally with lobulation, spiculation, vessel convergence, pleural indentation and mediastinal or hilar lymphonode involvement. Due to these signs of malignancy, 15 of 67 patients with PNLH in our center were firstly considered as lung cancer. Other auxiliary examinations such as blood examination also seemed unhelpful. In this study, hematological index such as white blood cell count, lymphocyte count, lymphocyte percentage and serum tumor markers were normal in most of the patients by referring to current normal range. Novel hematological indexes are needed to help distinguish PNLH from other diseases, and more innovative clinical studies focusing on this issue are required in the future. Some researchers reported that IgG4-positive plasma cells and the IgG4/IgG ratio were significantly increased in PNLH (5,20), but others suggested that PNLH did not show any convincing clinical features of IgG4 related disease or elevated serum IgG4 level (14).
By contrast, surgical resection is not only the diagnostic but also curative approach. Although some studies suggested the possibility of spontaneous regression of the remaining lesions with a lesion of PNLH resected (4,9), no evidence suggested that PNLH can regress without operation. The surgical approaches of PNLH included lobectomy and sublobar resection, but there was no reported study about the superiority of these two methods. In this study, we firstly compared perioperative data between lobectomy and sublobar resection groups. Sublobar resection showed significant advantages on operation time, blood loss, volume and duration of chest drainage after surgery and postoperative hospital stay. There also was statistical difference in the rate of VATS and lymphadenectomy. In theory, lymphadenectomy is not necessary in benign lesions. In our center, the rate of diagnostic accuracy of frozen section is very high and even more than 98%, especially in the benign lesions. In this study, all lesions were diagnosed by intraoperative frozen section examination as lymphoid hyperplasia, inflammatory disease or benign disease, so the diagnosis of frozen section is a fairly reliable indication to not performing lymphadenectomy. However, 19 patients were performed lymphadenectomy due to similar manifestations to lung cancer and lymphadenectasis on radiological exam. Although the relatively small sample size limited further statistical analysis to prove the safety and efficacy of sublobar resection more rigorously, sublobar resection in VATS was suitable to treat PNLH due to its advantages of ERAS and preferable long-term outcome. During our follow-up time, no disease related death and recurrence occurred.
Compared with lobectomy, sublobar resection is an alternative approach for the treatment of PNLH, which confers perioperative advantages and similar long-term prognosis. The diagnosis before the operation is still challenging which requires more clinical researches in the future and PNLH should be taken into consideration in the differential diagnosis of lung nodules.
Acknowledgements
Funding: This study was funded by Major science and technology projects of Zhejiang province (2014C03032), Key research project of traditional Chinese medicine science and technology plan in Zhejiang Province (2015ZZ007) and National Key R&D Program of China (2017YFC0113500).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Zhejiang University, School of Medicine (ID of ethics approval: 20181032).
References
- Saltzstein SL. Pulmonary Malignant Lymphomas and Pseudolymphomas: Classification, Therapy, and Prognosis. Cancer 1963;16:928-55. [Crossref] [PubMed]
- Kradin RL, Mark EJ. Benign lymphoid disorders of the lung, with a theory regarding their development. Hum Pathol 1983;14:857-67. [Crossref] [PubMed]
- Abbondanzo SL, Rush W, Bijwaard KE, et al. Nodular lymphoid hyperplasia of the lung: a clinicopathologic study of 14 cases. Am J Surg Pathol 2000;24:587-97. [Crossref] [PubMed]
- Kajiwara S, Sakai S, Soeda H, et al. Multifocal nodular lymphoid hyperplasia of the lung. J Thorac Imaging 2005;20:239-41. [Crossref] [PubMed]
- Judge EP, Abrahams J, Costigan D, et al. Pulmonary Nodular Lymphoid Hyperplasia presenting cavitating pulmonary nodules. Pathol Res Pract 2015;211:1006-9. [Crossref] [PubMed]
- Siemienowicz M, Simkin P, Straub A, et al. Locally invasive nodular lymphoid hyperplasia: radiological findings in a case of pulmonary and sinus disease. J Med Imaging Radiat Oncol 2009;53:554-7. [Crossref] [PubMed]
- Nakamura H, Miwa K, Haruki T, et al. Multifocal nodular lymphoid hyperplasia of the lung differently identified by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET). Thorac Cardiovasc Surg 2009;57:439-40. [Crossref] [PubMed]
- Karube Y, Chida M, Honma K, et al. Nodular lymphoid hyperplasia: rare case of lymphoproliferative disease in the lung. Gen Thorac Cardiovasc Surg 2009;57:324-7. [Crossref] [PubMed]
- Miyoshi S, Hamada H, Katayama H, et al. A case of pulmonary nodular lymphoid hyperplasia with a resected cavity, followed by spontaneous regression of the remaining lesions. Intern Med 2010;49:1617-21. [Crossref] [PubMed]
- Mitchell JD, Yu JA, Bishop A, et al. Thoracoscopic lobectomy and segmentectomy for infectious lung disease. Ann Thorac Surg 2012;93:1033-9; discussion 1039-40. [Crossref] [PubMed]
- Lin L, Hu D, Zhong C, et al. Safety and efficacy of thoracoscopic wedge resection for elderly high-risk patients with stage I peripheral non-small-cell lung cancer. J Cardiothorac Surg 2013;8:231. [Crossref] [PubMed]
- Yuan P, Cao JL, Huang S, et al. Sublobar Resection for Pulmonary Aspergilloma: A Safe Alternative to Lobectomy. Ann Thorac Surg 2017;103:1788-94. [Crossref] [PubMed]
- Sakurai H, Hada M, Oyama T. Nodular lymphoid hyperplasia of the lung: a very rare disease entity. Ann Thorac Surg 2007;83:2197-9. [Crossref] [PubMed]
- Bois MC, Sekiguchi H, Ryu JH, et al. No definite clinical features of immunoglobulin G4-related disease in patients with pulmonary nodular lymphoid hyperplasia. Hum Pathol 2017;59:80-6. [Crossref] [PubMed]
- Guinee DG. Jr. Update on Nonneoplastic Pulmonary Lymphoproliferative Disorders and Related Entities. Arch Pathol Lab Med 2010;134:691-701. [PubMed]
- Song MK, Seol YM, Park YE, et al. Pulmonary nodular lymphoid hyperplasia associated with Sjogren’s syndrome. Korean J Intern Med 2007;22:192-6. [Crossref] [PubMed]
- Roca B, Ferran G, Simon E, et al. Lymphoid hyperplasia of the lung and Evans' syndrome in IgA deficiency. Am J Med 1999;106:121-2. [PubMed]
- Franchi LM, Chin TW, Nussbaum E, et al. Familial pulmonary nodular lymphoid hyperplasia. J Pediatr 1992;121:89-92. [Crossref] [PubMed]
- Yilmaz U, Unsal I, Halilcolar H, et al. Nodular lymphoid hyperplasia of the lung: the role of positron emission tomography in diagnosis. Tuberk Toraks 2009;57:417-21. [PubMed]
- Guinee DG Jr, Franks TJ, Gerbino AJ, et al. Pulmonary nodular lymphoid hyperplasia (pulmonary pseudolymphoma): the significance of increased numbers of IgG4-positive plasma cells. Am J Surg Pathol 2013;37:699-709. [Crossref] [PubMed]


