
Co-expression of CD44/MyD88 is a poor prognostic factor in advanced epithelial ovarian cancer
Introduction
Epithelial ovarian cancer (EOC) is the leading cause of gynecologic cancer mortality worldwide (1). Due to no symptoms, EOC is usually diagnosed at a late stage. Although many tumors commonly relieve effectively after initial treatment, the survival outcome of EOC patients with metastatic and/or recurrent disease is still extremely poor (2,3). Due to the drug resistance and the limit on the efficacy of second round chemotherapy, the 5-year survival rate for EOC is only about 30% (4). This disappointing situation strongly suggests that it is necessary to develop novel therapeutic strategies for preventing or targeting chemoresistant recurrence, to improve survival.
Tumor-initiating cells or cancer stem cells (CSCs) can self-renew and generate differentiated cells (non-stem cells) to provide a cell reservoir and maintain the tumor and may be responsible for drug resistance and the primary source of recurrence (5). CSCs are capable to survive conventional treatments, which usually target fast dividing cells, and give rise to recurrent tumors that are more chemo-resistant and more aggressive (6,7). Current evidence suggests that the molecular characterization of EOC stem cells is CD44+/MyD88+ (8).
Tumors are heterogeneous and consist of multiple types of cancer cells, which exhibit different chemo-responsiveness. An important characteristic of CD44+/MyD88+ EOC stem cells, which differentiate them from the CD44−/MyD88− EOC cells, is the presence of a functional TLR4/MyD88 pathway. This pathway drives NF-κB activity and constitutively secretes pro-inflammatory cytokines, which confers paclitaxel resistance and plays a critical role in their own survival and tumor progression (9-12). Our previous study demonstrated that expression of TLR4 is in detected all ovarian tissues, and expression of MyD88 correlated with EOC chemoresistance and poor clinical outcome, which was detected in 77.1% of patients with EOC (10). CD44 is expressed in the majority of EOC. However, to date, investigation of CD44 has yielded conflicting results. Particularly, the role of CD44/MyD88 expressing in human ovarian cancer remains elusive.
Therefore, we focused on investigating the co-expression of CD44 and MyD88 in EOC tissues and the correlation with tumor progression, metastasis, and recurrence in patients with advanced EOC.
Methods
Tumour samples
A total of 138 patients who underwent surgery from 1999 to 2009 at the Sichuan Cancer Hospital were investigated in this study with an advanced stage EOC
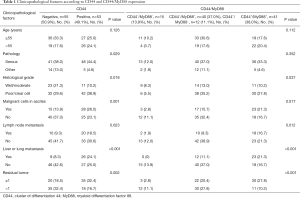
A total of 138 patients who underwent surgery from 2005 to 2015 from at Sichuan Cancer Hospital & institute were investigated in this study, including EOC tissue (n=108), normal ovarian tissue (n=10), benign cysts (n=10) and borderline tumors (n=10). This study included patients with primary EOC that was at International Federation of Gynecology and Obstetrics (FIGO) IIIc–IVa. Table 1 described the information on EOC patient age and tumor features, which was acquired from clinical and pathological data. Tumor stages were classified according to the standard proposed by the. Tissue was obtained before chemotherapy. All patients underwent 6–8 cycles paclitaxel/carboplatin (TP) combination chemotherapy after primary cytoreductive surgery. Cancers associated with germ cell tumor, sex cord-stromal tumors, or secondary tumors were excluded. The diagnosis, histological type and grade of all tumor tissues were confirmed by two pathologists. The tissue samples were obtained by ovarian resection, then fixed by formalin and embedded in paraffin for immunohistochemistry.
Full table
Ethics approval and consent to participate
Ethical approval for this project was obtained from our Internal Ethics Committee. Written informed consent was obtained from all subjects. All methods were carried out in accordance with the approved guidelines.
Immunohistochemistry
The tumor tissue (4-µm), which was continuously sliced with paraffin wax, was dewaxed in xylene and then hydrated in a series of ethanol, and the antigen was repaired by using autoclave oven technique. To quench the activity of endogenous peroxidase, the slides were then placed in a dye dish containing 0.3% H2O2 for 30 minutes and rinse three times with PBS. 100–200 µL of 5% BSA in PBS was added to the circumscribed areas and incubated for 20 min to avoid non-specific background. To keep the tissue or cell from drying out, the whole procedure was performed in a moisture chamber at room temperature. Next, the primary antibody [rabbit anti-human CD44, or MyD88 Abs (4–5 µg/mL); MyD88 Abs (4–5 µg/mL; Epitomics and Abcam, USA)] incubate overnight in 4 °C, then rinse three times with PBS, and specimens with biotin-resistant rabbit IgG (5 µg/mL; SANTA, USA) and horseradish peroxidase chain mildew resistant biotin protein (4MG/ML) were incubated at 37 °C for 50 minutes. The DAB (2.5 mg 3,3'-two amino-benzidine in 5 mL 0.1 mol/L Tris) is used for color rendering. Add 25 µL 0.03% H2O2 to the color source before use. The glass slides were dyed with Hematoxylin and loaded in glycerin gel after washing with double steaming water and then
Evaluation of immunohistochemical findings
Two pathologists, who did not know the clinical and results data, independently diagnosed each slide. The use of digital cameras (Olympus IX71 inverted fluorescence microscope and analysis image capture software) captured different staining density regions of high-power fields (×400), including the higher, middle, low, and negative staining region. The photo is printed on plain paper and the grid is drawn on it. We calculated percentage of positive staining tumor cells in an average of 2,000 tumor cells per tumor (range, 1,500–2,500). Afterwards, according to the grade of 0–4 scale (0: no staining; 1+: ≤10%; 2+: 11–30%; 3+: 31–50%; 4+: >50%), to score the percentage of CD44 or MyD88 positive tumor cells. The staining intensity score: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. Multiplies he percentage and staining intensity scores to produce a “combination” score: 0, negative (–); 1, 2, slight positive (+); 3, 4, 6, moderately positive (++); 9, 12, strongly positive (+++) (13).
Statistical analysis
SPSS 17.0 software was used for analysis. Use the Pearson X2 or Fisher’s exact test to compare qualitative variables. DFS was defined as the time from the date of surgery to the first day of detecting recurrence. The date of death or last follow-up was used if there was no recurrence. OS was defined as time interval between the date of the operation and last follow up or death. The median follow-up period for DFS and OS from initial surgery was 5 years. The Kaplan-Meier curve was used to estimate DFS and OS, which was compared by the log-rank test. The recurrence and death time were analyzed by cox proportional hazards model with univariate and multivariate analyses. The risk ratio (HR) between the prognostic group and its 95% confidence interval was calculated. The probability value (P) <0.05 is considered to be statistically significant.
Results
Prevalence of CD44+/MyD88+ cells in EOC tissues
The first purpose was to identify the prevalence of CD44+/MyD88+ cells in paraffin-embedded EOC tumor sections obtained before initiation of chemotherapy. The expression of CD44 varied substantially, from no expression to strong expression. Immunohistochemical staining showed that 53 of the 108 patients’ samples was detected CD44 positive (Figure 1). There is no or very weak expression of CD44 in the normal ovarian epithelium (2 of 10, 20.0%), benign cysts (2 of 10, 20.0%), borderline tumors (3 of 10, 30.0%). In addition, 41 (38.0%) cases showed a co-expression of CD44/MyD88. In the co-expression analysis, EOC samples with CD44-positive expression frequently showed high levels of MyD88 (P=0.007). CD44 and MyD88 expression were relevant through Pearson and Spearman correlation coefficient analysis (P=0.026, P=0.023, respectively). For comparison, patients were classified into three groups, according to the prevalence of CD44 and MyD88 expression in tumors: CD44−/MyD88−, CD44−/MyD88+ or CD44+/MyD88−, and CD44+/MyD88+.
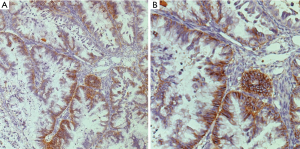
Clinicopathological significance of CD44/MyD88
The relationship between the distribution of CD44/MyD88 expression and EOC clinicopathological features is shown in Table 1. There was significant correlation between CD44 expression and histological type, histological grade, malignant cells in ascites, liver or lung metastasis, lymph node metastasis and residual tumor (P<0.05). Furthermore, CD44/MyD88 co-expression significantly correlated with histological grade, malignant cells in ascites, liver or lung metastasis, lymph node metastasis and residual tumor (P<0.05). No significant correlation between CD44/MyD88 expression and age.
Clinicopathological parameters and patient survival in EOC
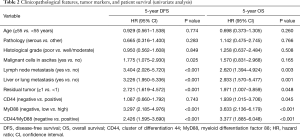
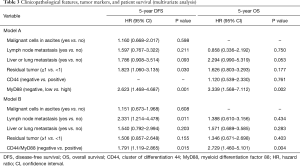
At 5-year follow-up, the recurrence rate was 58.3% (63 patients), and mortality was 37.9% (41 patients). In univariate analysis, ascites malignant cells, liver or lung metastasis, lymph node metastasis and residual tumor were important factors associated with DFS and OS (P<0.05). Patient age, pathology and histological grade had no correlation with DFS or OS (Table 2). Independent prognostic factors were identified through multivariate analysis. However, these clinicopathological parameters were not identified as an independent risk factor for either recurrence or death on multivariate analysis (Table 3).
Full table
Full table
CD44+/MyD88+ correlation with patient survival
We evaluated the effects of CD44 and CD44/MyD88 expression on the survival of patients with EOC. Compared with a negative CD44 expression, a positive expression of CD44 had no significant impact on DFS (median DFS: 18.76 vs. 20.96 months; log-rank P=0.743; Figure 2A). However, a positive CD44 expression was involved in the worse OS (median OS: 25.23 vs. 42.91, log-rank P=0.041; Figure 2B). A significantly poorer DFS (log-rank P<0.001, Figure 2C) and OS (log-rank P<0.001, Figure 2D) was found in the patients with co-expression of CD44/MyD88.
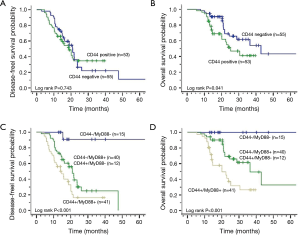
In univariate analysis of Table 2, there was no significant correlation between CD44 expression and DFS. MyD88 expression (HR: 3.297; 95% CI: 2.185–4.976; P<0.001) and CD44/MyD88 co-expression (HR: 2.426; 95% CI: 1.595–3.690; P<0.001) significantly influenced DFS. In addition, CD44 expression (HR: 1.939; 95% CI: 1.015–3.706; P=0.045), MyD88 expression (HR: 3.633; 95% CI: 2.136–6.179; P<0.001), and CD44/MyD88 co-expression (HR: 3.377; 95% CI: 1.885–6.048; P<0.001; Table 2) was particularly associated with poor OS. As the co-expression CD44/MyD88 includes both CD44 and MyD88 information, we established two models, respectively. In multivariate analysis, MyD88 expression particularly related to poor DFS (adjusted HR: 2.623; 95% CI: 1.468–4.687; P=0.001) and OS (adjusted HR: 3.339; 95% CI: 1.568–7.112; P=0.002) (Table 3 model A). As Table 3 model B shown, co-expression of CD44/MyD88 also significantly related to poor DFS and OS (adjusted HR: 1.791; 95% CI: 1.119–2.865; P=0.015; adjusted HR: 2.729; 95% CI: 1.460–5.101; P=0.004, respectively).
Discussion
CD44, as a cell surface receptor, is associated with cell signaling, differentiation, adhesion, proliferation, migration and angiogenesis, which are important properties for normal and cancerous cell function. Various epithelial malignancies, including ovarian cancer, often expressed CD44 which is a potential marker for the identification of CSCs (14-16). While CD44 is absent or very low in normal epithelial ovarian cells (17,18), CD44 has become a specific molecular marker for normal stem-like epithelial cells in the distal end of the fallopian tube (19). CD44 was frequently overexpressed in EOC play complex roles in tumor progression and metastasis. Expression of CD44 and specific isoforms in epithelial ovarian carcinoma has remained a controversial topic. Some studies have shown that CD44 expression have a significant correlation with metastasis and survival outcome (20-23), while in contrast, other studies have found no association (17,24-26). Additionally, other studies have indicated that high CD44s expression is a factor in improving prognosis of ovarian cancer (8,18,27). In this study, our data showed that CD44 may be an important molecular marker for poor prognosis, which associated with histological type and grade, residual tumor, metastasis, and 5-year survival. However, the differences of each study were not surprising because technical factors, including the use of various antibodies and detection methods, could exist a certain distinction. The other reason was that the cohorts of EOC patients examined in different studies were highly heterogeneous.
Recently, Mor et al. reported a distinctive phenotype of EOC stem cells characterized by CD44+/MyD88+, and confirmed the functionality of the TLR4/MyD88 pathway only in the CD44+ cell population (8). Toll like receptors (TLRs), particularly the TLR4 signaling pathway, are involved in tissue renewal and repair, the control of infection, and may correlated with tumor formation (28-30). MyD88, as a joint protein, is a critical component of TLR4 pathway. The activation of TLR4/MyD88 pathway leads to downstream activation of the NF-κB signaling pathway, cytokine production and chemo-resistance. CD44+ EOC cells express the TLR4/MyD88 pathway may promote the process of repair/differentiation triggered by the CSCs. However, the investigation about the association between the co-expression CD44/MyD88 and cancer clinicopathological factors, prognostic significance, has not been done in clinical samples. It is noticed that MyD88 expression significantly decreased with the differentiation of ovarian CSC in ex vivo manipulation (31). This compared similarly to our findings, showing that there was significant relation between CD44 and MyD88 expression in EOC patients.
Hyaluronan (HA) induces CD44 interaction with TLR-4 signaling pathway, like “cross-talk”, stimulating the production of cytokine/chemokine production in a CD44-specific and MyD88-dependent manner, resulting in the adhesion, migration, and invasion of EOC cells (32,33). Ovarian cancer tumors consist of CSCs (CD44+/MyD88+), progenitor cells (CD44−/MyD88+ or CD44+/MyD88−) and fast dividing cells (CD44−/MyD88−), which constitutes the tumor heterogeneity (27). Our findings suggested that co-expression of CD44/MyD88 promotes EOC metastasis and progression, and is an independent and significant poor prognostic factor.
Although 70% of ovarian cancers is effective for initial treatment (surgery followed with TP combination chemotherapy), but most cases frequently recur and develop chemotherapeutic resistance. It has been demonstrated that paclitaxel selectively induce cell death in CD44−/MyD88− EOC cells but has a pro-survival effect and enhances self-renewal in the pleuripotent and chemoresistant CD44+/MyD88+ EOC stem cells (34). Fully recognize characteristic Molecular marker, like CD44/MyD88, which was associated with metastasis, recurrence and drug resistance, is helpful for designing a more strategical EOC therapeutics. Our results highlight the need to identify the EOC CD44+/MyD88+ patients, who should not receive paclitaxel chemotherapy. The reason is that CD44+/MyD88+ EOC has paclitaxel resistance, and more importantly, it can enrich more aggressive CSCs. Moreover, it has HA-grafted particle clusters loaded with Mitomycin C as selective nanovectors for cancers, which might be suitable for future treatment of many CD44-expression tumors (35,36). Hyaluronan-CD44 antagonists provide another reasonable therapy for eliminating the characteristics of these cells (37). Our previous study reported that AO-I could significantly enhance the sensitivity of MyD88-positive EOC cells to chemotherapy of paclitaxel by blocking TLR4/MyD88 signaling. These therapeutic approaches could be a promising strategy for targeting at CD44/MyD88 co-expression EOC (38).
Taken together, CD44+/MyD88+ was an useful and important marker, which had contributed to tumor progression and poor prognosis in patients with EOC, as well as a potentially effective therapeutic target for prevention and treatment of metastasis and recurrence. Based on these data, we propose that the mode of management for EOC patients should take into consideration the tumor's molecular phenotype.
Acknowledgements
Funding: Sichuan Key Research and Development Project from Sichuan Provincial Science and Technology Department (19ZDYF0716).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our Internal Ethics Committee and written informed consent was obtained from all patients.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Davidson B, Reich R, Trope CG, et al. New determinates of disease progression and outcome in metastatic ovarian carcinoma. Histol Histopathol 2010;25:1591-609. [PubMed]
- Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res 2002;107:99-118. [PubMed]
- Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 2011;61:183-203. [Crossref] [PubMed]
- Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell 2006;124:1111-5. [Crossref] [PubMed]
- Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle 2008;7:1371-8. [Crossref] [PubMed]
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11. [Crossref] [PubMed]
- Alvero AB, Chen R, Fu HH, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle 2009;8:158-66. [Crossref] [PubMed]
- Szajnik M, Szczepanski MJ, Czystowska M, et al. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene 2009;28:4353-63. [Crossref] [PubMed]
- Zhu Y, Huang JM, Zhang GN, et al. Prognostic significance of MyD88 expression by human epithelial ovarian carcinoma cells. J Transl Med 2012;10:77. [Crossref] [PubMed]
- Kim KH, Jo MS, Suh DS, et al. Expression and significance of the TLR4/MyD88 signaling pathway in ovarian epithelial cancers. World J Surg Oncol 2012;10:193. [Crossref] [PubMed]
- Huang B, Zhao J, Unkeless JC, et al. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene 2008;27:218-24. [Crossref] [PubMed]
- Hu Z, Gao J, Zhang D, et al. High expression of Lewis y antigen and CD44 is correlated with resistance to chemotherapy in epithelial ovarian cancers. PLoS One 2013;8:e57250. [Crossref] [PubMed]
- Saegusa M, Machida D, Hashimura M, et al. CD44 expression in benign, premalignant, and malignant ovarian neoplasms: relation to tumour development and progression. J Pathol 1999;189:326-37. [Crossref] [PubMed]
- Cho EY, Choi Y, Chae SW, et al. Immunohistochemical study of the expression of adhesion molecules in ovarian serous neoplasms. Pathol Int 2006;56:62-70. [Crossref] [PubMed]
- Kayastha S, Freedman AN, Piver MS, et al. Expression of the hyaluronan receptor, CD44S, in epithelial ovarian cancer is an independent predictor of survival. Clin Cancer Res 1999;5:1073-6. [PubMed]
- Cannistra SA, Abu-Jawdeh G, Niloff J, et al. CD44 variant expression is a common feature of epithelial ovarian cancer: lack of association with standard prognostic factors. J Clin Oncol 1995;13:1912-21. [Crossref] [PubMed]
- Sillanpää S, Anttila MA, Voutilainen K, et al. CD44 expression indicates favorable prognosis in epithelial ovarian cancer. Clin Cancer Res 2003;9:5318-24. [PubMed]
- Paik DY, Janzen DM, Schafenacker AM, et al. Stem-like epithelial cells are concentrated in the distal end of the fallopian tube: a site for injury and serous cancer initiation. Stem Cells 2012;30:2487-97. [Crossref] [PubMed]
- Cannistra SA, Kansas GS, Niloff J, et al. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res 1993;53:3830-8. [PubMed]
- Bourguignon LY, Zhu H, Zhou B, et al. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185(HER2) and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem 2001;276:48679-92. [Crossref] [PubMed]
- Volz Y, Koschut D, Matzke-Ogi A, et al. Direct binding of hepatocyte growth factor and vascular endothelial growth factor to CD44v6. Biosci Rep 2015;35. [Crossref] [PubMed]
- Preca BT, Bajdak K, Mock K, et al. A self-enforcing CD44s/ZEB1 feedback loop maintains EMT and stemness properties in cancer cells. Int J Cancer 2015;137:2566-77. [Crossref] [PubMed]
- Speiser P, Wanner C, Breitenecker G, et al. CD-44 is not involved in the metastatic spread of ovarian cancer in vivo. Anticancer Res 1995;15:2767-9. [PubMed]
- Sanchez Lockhart M, Hajos SE, Basilio FM, et al. Splice variant expression of CD44 in patients with breast and ovarian cancer. Oncol Rep 2001;8:145-51. [PubMed]
- Ross JS, Sheehan CE, Williams SS, et al. Decreased CD44 standard form expression correlates with prognostic variables in ovarian carcinomas. Am J Clin Pathol 2001;116:122-8. [Crossref] [PubMed]
- Rodríguez-Rodríguez L, Sancho-Torres I, Mesonero C, et al. The CD44 receptor is a molecular predictor of survival in ovarian cancer. Med Oncol 2003;20:255-63. [Crossref] [PubMed]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004;118:229-41. [Crossref] [PubMed]
- Pull SL, Doherty JM, Mills JC, et al. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA 2005;102:99-104. [Crossref] [PubMed]
- Chen R, Alvero AB, Silasi DA, et al. Cancers take their Toll--the function and regulation of Toll-like receptors in cancer cells. Oncogene 2008;27:225-33. [Crossref] [PubMed]
- d'Adhemar CJ, Spillane CD, Gallagher MF, et al. The MyD88+ phenotype is an adverse prognostic factor in epithelial ovarian cancer. PLoS One 2014;9:e100816. [Crossref] [PubMed]
- Sacks JD, Barbolina MV. Expression and Function of CD44 in Epithelial Ovarian Carcinoma. Biomolecules 2015;5:3051-66. [Crossref] [PubMed]
- Bourguignon LY, Wong G, Earle CA, et al. Interaction of low molecular weight hyaluronan with CD44 and toll-like receptors promotes the actin filament-associated protein 110-actin binding and MyD88-NFkappaB signaling leading to proinflammatory cytokine/chemokine production and breast tumor invasion. Cytoskeleton (Hoboken) 2011;68:671-93. [Crossref] [PubMed]
- Alvero AB, Craveiro V, Holmberg J, et al. Abstract 3471: Paclitaxel selects and enriches for CD44+/MyD88+ ovarian cancer stem cells. Cancer Res 2012;72:3471. [Crossref]
- Bachar G, Cohen K, Hod R, et al. Hyaluronan-grafted particle clusters loaded with Mitomycin C as selective nanovectors for primary head and neck cancers. Biomaterials 2011;32:4840-8. [Crossref] [PubMed]
- Li SD, Howell SB. CD44-targeted microparticles for delivery of cisplatin to peritoneal metastases. Mol Pharm 2010;7:280-90. [Crossref] [PubMed]
- Slomiany MG, Dai L, Tolliver LB, et al. Inhibition of Functional Hyaluronan-CD44 Interactions in CD133-positive Primary Human Ovarian Carcinoma Cells by Small Hyaluronan Oligosaccharides. Clin Cancer Res 2009;15:7593-601. [Crossref] [PubMed]
- Huang JM, Zhang GN, Shi Y, et al. Atractylenolide-I sensitizes human ovarian cancer cells to paclitaxel by blocking activation of TLR4/MyD88-dependent pathway. Sci Rep 2014;4:3840. [Crossref] [PubMed]


