Surgery for advanced non-small cell lung cancer patient after epidermal growth factor receptor tyrosine kinase inhibitor neoadjuvant therapy
Introduction
Upon the arrival of targeted therapy era for subtypes of lung cancer according to the oncogenic driver mutation, epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) that target EGER mutations found in NSCLC patients has the potential to turn this deadly disease to an operable status before surgery. Gefitinib is an orally bioavailable, synthetic anilinoquinazoline that selectively and reversibly binds the intracellular ATP-binding site of the EGFR tyrosine kinase, one of the commonly found oncogenic driver mutation for NSCLC. Several randomized clinical trials have shown its efficacy as first-line treatment in patients with tumors harboring EGFR gene mutations (1). Some patients with inoperable advanced NSCLC have shown changed to be operable status after gefitinib treatment (2,3). Despite dramatic efficacy of EGFR-TKI for those EGFR mutant patients, the median time to progression is about 1 year, before the resistance mutation occur (4). Before the patient developing drug resistance to EGFR-TKI, if the radical surgery could be performed, then the patients may avoid the risk of progression and process a prolonged survival. We had performed radical surgery for 10 patients stage IIIA with no metastasis, (N2 metastasis or great vessels invasion, according to the AJCC 7th edition) after response to EGFR-TKI (gefitinib) neoadjuvant therapy. It suggested that this type of patients may benefit from EGFR-TKI neoadjuvant therapy. We presented our experiences of EGFR-TKI (gefitinib) neoadjuvant therapy in advanced non-small cell lung cancer (NSCLC).
Methods
Inclusion criteria
This study was performed retrospectively. From 2014 to 2016, 10 patients who are diagnosed as advanced stage NSCLC (N2 metastasis or great vessels invasion) were recruited in this study. The metastasis status of these patients was confirmed by endobronchial ultrasound (EBUS) or positron emission tomography-computed tomography (PET-CT) scan. Great vessels invasions were suggested by contrasted CT or magnetic resonance imaging (MRI) scan. The pathology and the EGFR mutations were confirmed by the needle biopsy under the guided CT scan or E-BUS. After responded to neoadjuvant therapy of gefitinib (250 mg/kg po. qd.), all patients responded to the EGFR-TKI with the tumor masses and N2 lymph nodes shrinkage or without invasion of the great vessels. This study was approved by the Ethical Committee of Shanghai Pulmonary Hospital (SHFK-18-01-042) and received the consents from all patients included in this study.
Preoperative assessment
Preoperative assessments according to the guideline of Thoracic Society including thoracic computed tomography (CT), brain MRI and F18-fluorodeoxyglucose positron emission tomography (FDG-PET) or bone ECT scan which were performed to establish the diagnosis of the pulmonary lesions and absence of hepatic, adrenal, bone or brain metastases. E-BUS or PET-CT was performed to confirm the N2 lymph node status and MRI were used to determine the status of the great vessel invasions.
Postoperative management
Tumor specimens from NSCLC patients were examined to establish the diagnosis of the tumor lesions. And involved lymph nodes were examined to determine the status of metastasis postoperatively. Postoperative follow-ups were conducted every 3 months since surgery till 12 months later, followed by check up every 6 months from 12 to 24 months post-operation, and continued with yearly check up. Standard follow-up consists chest X-ray or CT, ultrasound, laboratory testing including measurement of tumor markers, and clinical examination. The patients information, including sex, age, smoking history, clinical (pretreatment) stage, adverse effects of gefitinib monotherapy, duration of gefitinib administration, preoperative clinical stage, surgical procedure, morbidity and mortality of surgery, histology, pathologic stage, EGFR mutation status, postoperative therapy, survival time, recurrence, and cause of death, were collected during the follow up study. Neoadjuvant therapy of gefitinib was continued until the tumor progresses or developed resistance to the treatment. Other alternative adjuvant therapies were conducted to prolong the lifespan of the patients.
Results
All the patients recruited in this study have been examined for EGFR mutation prior to the treatment (Table 1). Preoperative stage of the lesions were established with thoracic CT, brain MRI and FDG-PET or bone ECT scan. E-BUS or PET-CT was performed to confirm the N2 lymph node status and MRI were used to determine the status of the great vessel invasions. The results were listed in Table 1. Among these patients, all of them received successful radical surgeries (complete resection of the tumor with systematic lymphadenectomy) with 3 cases of right upper lobe lobectomy, 2 case of right lower lobe lobectomy, 2 cases of left upper lobe lobectomy, 2 cased of left lower lobe lobectomy and 1 case of left pneumonectomy (Table 2). One patient passed away 7 days postoperatively due to respiratory failure. The necropsy analysis of the patient indicates the replacement of tumors by fibrotic scar tissue, and concentration of focal residual tumors limited in areas of fibrotic stroma as well as lymphocyte infiltration. Adjuvant therapy of gefitinib was applied for every patient immediately after the operation. Follow-up study of these consented patients were conducted with contrasted CT scan, ultrasonography, bronchoscope and molecular examination of EGFR mutation and others for at least 8 months (8–30 months, median time: 24 months) (Figure 1). The treatment strategy for each patient and clinical outcome is shown in Table 2. Among all the patients examined, the progression-free survival (PFS) of them is 14 months and the overall survival is up to 36 months (Figure 2).
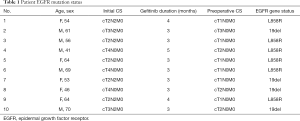
Full table
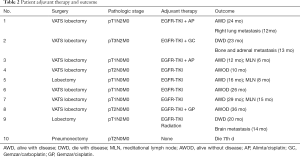
Full table
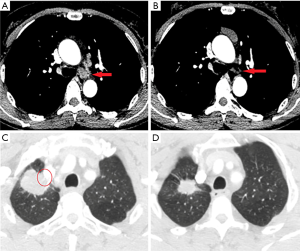
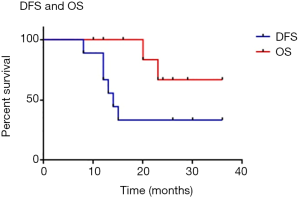
Our study showed that EGFR-TKI neoadjuvant therapy is feasible and effective, and this treatment strategy in combination with surgery may improve the survival rate of advanced NSCLC patients with known EGFR mutation, especially to those T4 and N2-positive lesions. This indicates that EGFR-TKI neoadjuvant therapy may lead to the achievement of the radical surgery of the patients with prolonged survival.
Discussion
Perioperative sequential or concurrent chemoradiotherapy (CRT) are standard treatment and part of patients could benefit moderately on respect of progression-free survival (PFS), overall prognosis of locally advanced patients is still quite poor. Standard of chemotherapy combinations, such as cisplatin/gemcitabine, cisplatin/docetaxel, carboplatin/paclitaxel, and cisplatin/paclitaxel, have been used for the treatment of advanced NSCLC in the clinic, which prolongs a median overall survival rate of around 8–10 months (5-7). With the discovery of oncogenic driver mutations that can be used for targeted therapy, EGFR-TKI have been proved to be able to convert advanced NSCLC to an operable status after the neoadjuvant treatment among those patients harboring EGFR mutations, including T790M, L858R and exon19 deletion. Since the progression of NSCLC tumors depend on these oncogenic driver mutations, EGFR-TKI has shown to be extremely effective in more than 70% of advanced NSCLC with EGFR mutations (4). Several cases report that patients with advanced stage undergo EGFR-TKI neoadjuvant therapy suggested that it could downstage the tumor status (8,9). But no further follow up study was conducted. In this study, we aimed at follow up study of the advanced stage patients with EGFR mutation after the surgery with EGFR-TKI gefitinib neoadjuvant treatment. After the EGFR-TKI neoadjuvant therapy, these patients regained the opportunity for fully recovery. In this study, these patients underwent salvage surgeries with the response to the EGFR-TKI neoadjuvant therapies. The dramatic results suggested a 14 months’ progression free survival and OS was up to 36 months. Similarly, in previous study, it is reported that the salvage surgery for advanced NSCLC after response to gefitinib in 9 cases (2). In that report, the PFS was 6 months and OS was up to 32 months. The potential reason of our cases have longer PFS might be the reduced amount of circulating tumor cells (CTCs) or residual tumor cells by EGFR-TKI treatment after the surgeries, thus increasing the cure rate of these advanced staged patients. Another possibility could be higher levels of lesions including stage IV lesions were included in the other study (2).
Consistent with our results, there are several other case reports suggested improved OS after EGFR-TKI treatment and down-staging of advanced NSCLC to operable status (10,11). The pathology of the lesions and the lymph nodes suggested the replacement of tumors by fibrotic scar tissue, and concentration of focal residual tumors limited in areas of fibrous stroma and lymphocyte infiltration. On the other hand, neoadjuvant chemotherapy may downstage the advanced NSCLC, but the scaring tissue and impaired physical condition may lead to a higher rate of surgical complications and mortality. As to the patient who died 7 days postoperatively because of respiratory failure, it suggested that the EGFR-TKI neoadjuvant therapy might also have an adverse effect on the physical condition similar as the neoadjuvant chemotherapy. EGFR-TKI will affect the blood supply and lead to the necrosis of the tumor and formation the scaring tissue, which may be a challenge for the surgery. According to our experience, the resection of the lesion is feasible for VATS procedure, while the lymphadenectomy is the difficult part for the VATS surgery. It requires patience and the subtle handling to finish the procedure. For those patients with advanced ages, bad physical conditions and the complicated surgical procedure should be taken extra caution while put on EGFR-TKI neoadjuvant therapy. In this study, the patient aged 70 who received pneumonectomy and lymphadenectomy had an impaired pulmonary function (FEV1: 1.75 L, 54% of the predicted value) with a 40-year of smoking history.
One shortcoming of this study is that it is a single arm treatment investigation without comparing the EGFR-TKI neoadjuvant therapy with a standard chemotherapy regimen, such as gemcitabine/carboplatin and others. However, our study showed that EGFR-TKI neoadjuvant therapy is feasible and effective, along with the adjuvant therapy after surgery may improve the survival of advanced stage patients including stage IIIA NSCLC patients. It also showed that those stage IIIA-T4 patients had better outcomes than those of stage IIIA-N2. This suggests that neoadjuvant therapy of gefitinib may be widely used for stage IIIA-T4 patients to obtain an opportunity of radical surgery.
The field of the development of EGFR inhibitors has progressed rapidly in recent years. In addition to the first generation of reversible competitive EGFR inhibitors that targeting EGFR mutations including L858R and Del19 mutation, such as gefitinib and erlotinib, the second-generation covalent irreversible EGFR inhibitors such as Afatinib and Neratinib are also used in the clinic. Recently, the third generation EGFR inhibitor osimertinib has also been approved as the first line treatment by FDA at USA for the metastatic NSCLC with most common EGFR mutations. And other inhibitors against other mutations are also under development. Forde et al. suggested that neoadjuvant nivolumab was associated with few side effects, did not delay surgery, and induced a major pathological response in 45% of resected tumors (12). It is expected that in the near foreseeable future, with rapid advances of precision medicine development, some advanced staged NSCLC patients would be provided the chances for radical surgery and obtained a promising outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethical Committee of Shanghai Pulmonary Hospital (SHFK-18-01-042) and received the consents from all patients included in this study.
References
- Azzoli CG, Baker S Jr, Temin S, et al. American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2009;27:6251-66. [Crossref]
- Hishida T, Nagai K, Mitsudomi T, et al. Salvage surgery for advanced nonsmall cell lung cancer after response to gefitinib. J Thorac Cardiovasc Surg 2010;140:e69-71. [Crossref] [PubMed]
- Takamochi K, Suzuki K, Sugimura H, et al. Surgical resection after gefitinib treatment in patients with lung adenocarcinoma harboring epidermal growth factor receptor gene mutation. Lung Cancer 2007;58:149-55. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Azzoli CG, Temin S, Aliff T, et al. 2011 Focused update of 2009 American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2011;29:3825-31. [Crossref] [PubMed]
- Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii56-64. [Crossref] [PubMed]
- Wang Q, Wang H, Li P, et al. Erlotinib-based perioperative adjuvant therapy for a case of unresectable stage IIIA (N2) nonsmall cell lung cancer. Am J Med Sci 2010;340:321-5. [Crossref] [PubMed]
- Shen H, Zhong X, Ge XQ, et al. Surgical resection of lung adenocarcinoma without EGFR mutation after neoadjuvant gefitinib treatment. Clin Respir J 2010;4:192-3. [Crossref] [PubMed]
- Funakoshi Y, Takeuchi Y, Maeda H. Pneumonectomy after response to gefitinib treatment for lung adenocarcinoma. Asian Cardiovasc Thorac Ann 2013;21:482-4. [Crossref] [PubMed]
- Provencio M, López-González A, Almagro E, et al. Use of a tyrosine kinase inhibitor as neoadjuvant therapy for non-small cell lung cancer: A case report. Respir Med Case Rep 2013;9:8-10. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]


