Use of lung-protective strategies during one-lung ventilation surgery: a multi-institutional survey
Introduction
Acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) are often devastating complications after major lung resections. The incidence ARDS and ALI after major lung resections has been reported to range from 2% to 4% in large cohort studies (1-3). When they occur, they can be associated with a 50–70% mortality (1-3). Strong evidence exists supporting the use of lung-protective ventilation (LPV) strategies in the setting of ARDS and ALI (4-6). The central element of these LPV strategies has been shown to be the use of low tidal volumes (TV) defined as 4–6 cc/kg of predicted body weight (3,7). LPV strategies also encompass lower peak airway pressures (PAP) in order to reduce barotrauma, higher positive end-expiratory pressure (PEEP) in order to reduce atelectotrauma and lower fractions of inspired oxygen (FiO2) in order to reduce oxygen toxicity (8-10). LPV has been shown to reduce mortality and increase ventilation-free days in patients with ARDS and ALI in the intensive care setting (4). Recent evidence has further demonstrated that minimization of TV as a lung-protective strategy improves respiratory outcomes in anesthetized patients at risk of pulmonary complications undergoing abdominal surgery and ICU patients even in the absence of pre-existing ARDS or ALI (11,12). There has been increasing interest in determining whether intra-operative LPV strategies may reduce respiratory complications and/or mortality following thoracic surgery requiring one-lung ventilation (OLV). Traditionally, concerns about the deleterious effects of intra-operative acidosis, hypoxia and pulmonary shunting resulted in OLV being performed with high TVs, high FiO2 and low PEEP (10,13). Recent studies have challenged these conventions and have shown that LPV during OLV surgeries is associated with a reduction in inflammatory markers as well as clinical surrogates of pulmonary dysfunction post-operatively (10,13,14). Thus, although no direct evidence exists demonstrating a reduction in respiratory complications or mortality with use of intra-operative LPV strategies during OLV surgeries, there has been a call to adopt LPV in this setting (7,10,15). Little is known about LPV practices in the setting of OLV surgeries. Our primary objective was to assess the state of practice as well as the perspectives of anesthesiologists regarding LPV during elective OLV surgeries in high-volume academic centres. Our secondary objectives were to assess the barriers to implementation of LPV strategies during elective OLV cases and to determine the factors that predict use of LPV by anesthesiologists during OLV. These are the first critical steps to a knowledge translation process.
Methods
A cross-sectional survey was designed, piloted and refined by a multi-disciplinary team comprising of thoracic surgeons, anesthesiologists, critical care physicians and clinical epidemiologists. The survey development process began with item generation by the multi-disciplinary team followed by multiple (8) iterations of piloting to allow for item reduction (from 60 questions down to 32) and testing for clinical sensibility as well as face, content and construct validity. The Dillman or tailored design method was used to guide the development, administration and distribution of this survey (16). After institutional research ethics board approvals, this survey was distributed electronically to all anesthesiologists who perform OLV surgeries in 3 high-volume Canadian tertiary centers. Anesthesiologists who perform OLV surgeries at each center were identified by each department of anesthesiology. The number of lung cancer operations in these centres ranged from approximately 205 to 600 in the 2010–2011 fiscal year, with additional OLV cases being performed for benign disease (17). Our initial plan was to use this 3-center survey as a platform to launch a broader national survey. These 3 centers were chosen because we anticipated that, due to their academic and high-volume nature as well as their location in the same province (within 200 kilometres of each other), they would result in a relatively homogeneous reference standard for responses from academic and non-academic OLV anesthesiologists across the country. Thus, we felt it important to report this unanticipated heterogeneity. For univariate analysis, normally distributed continuous data were analyzed using independent sample t-tests, while the non-parametric Mann-Whitney U test was utilized where data were not normally distributed. Fisher’s exact tests were used for univariate analysis of categorical data. Multivariable logistic regression with backward stepwise modelling was used to analyze the predictors of attempts to minimize TV by anesthesiologists during OLV. Predictors were theory-driven (i.e., factors that the multi-disciplinary team believed would influence an anesthesiologist to actively minimize TVs) and were chosen a priori. A 2-sided alpha of 0.05 was used for all tests of significance. Analyses were performed using SPSS/PASW v.20 (IBM Corporation, Armonk, NY, USA).
Results
Seventy-five (63%) of 120 eligible respondents completed the survey. Completion rates ranged from 51% to 74% among the centers. Overall, the respondents had been practicing as anesthesiologists for a mean of 15.8±10.3 years. The respondents had a varied practice with OLV surgeries comprising a mean of 8.6% (6.9%) of their caseload in the year preceding the survey. All following reported percentages for categorical data represent the analyzable dataset (i.e., excluding missing responses). Males comprised 64% (n=36) of respondents; 19 respondents (25%) did not specify their sex. Only 13% (n=10) reported any formal fellowship training in cardiothoracic anesthesia and only 25% (n=19) reported ever having worked as a critical care physician in an intensive care unit (ICU). Approximately 61% (n=46) perceived that the LPV evidence in the ICU setting was very or somewhat applicable to the intra-operative setting whereas 7% (n=5) perceived a lack of applicability. The remaining respondents were undecided about the applicability of the ICU evidence to the intra-operative setting. Approximately 39% and 47% of respondents used predominantly Pressure-controlled and Volume-controlled ventilation modes, respectively; the rest reported equal use of both ventilation modes.
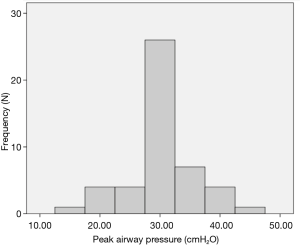
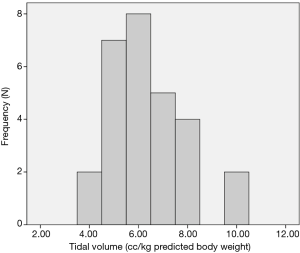
Although the literature in the ICU setting focuses on minimizing TV as the primary strategy of LPV, most respondents (89%, n=50 out of 56 respondents) reported focusing on minimizing PAP as their primary strategy of intra-operative LPV. Respondents reported trying to keep PAP below a mean of 30.3 (5.8) cmH2O (median 30 cmH2O, interquartile range, 30–35) (Figure 1). Only 64% (n=37 out of 58 respondents) reported actively trying to minimize TV. Respondents reported trying to keep TV below a mean of 6.4 (1.5) cc/kg predicted body weight (median 6 cc/kg, interquartile range, 5–7) (Figure 2). All respondents reported that their practices did not change with VATS with respect to minimization of PAP and TV. Approximately 73% (n=41 of 56) reported their PAP and/or TV minimization strategies would be very similar for bilateral lung ventilation cases. When asked to rate in order of importance what defines LPV, the aggregate order placed minimization of PAP as most important followed by minimization of TV (Figure 3).
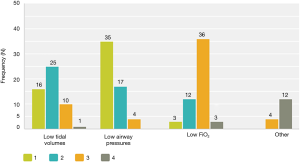
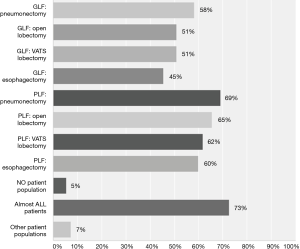
While 32% (n=17 of 54) were unsure whether the current evidence supported the use LPV during OLV, 67% (n=36 of 54) believed that the evidence favoured their use during OLV. Thus, respondents were either unsure about the evidence or thought it favoured the use of LPV during OLV. Figure 4 depicts the populations that respondents felt would benefit from LPV. In general, it appeared that respondents believed that LPV practices would benefit patients with poorer baseline lung function and those with larger pulmonary resections.
Since anesthesiologists were more likely to focus on minimizing PAP rather than TV, we were interested in determining which factors predicted active attempts to minimize TVs during OLV surgeries. On univariate analyses, perceived clinical (P=0.047) and institutional (P=0.002) barriers were the only significant predictors such that they predicted reduced attempts to minimize TV (Table 1). The main clinical barriers to minimizing TVs reported were: concern about atelectasis (40%, n=20), concern about hypoxia and acidosis (36%, n=18), as well as the sense that there were too many other competing demands intra-operatively (14%, n=7). The main institutional barriers (i.e., customs and practice patterns in a workplace) reported were: a lack of a protocol to implement low TV in the context of LPV (24%, n=12) and perceived surgeon/institutional bias against low TV during OLV (10%, n=5). However, 76% (n=39/51) of the anesthesiologists reported no institutional barriers to implementation.

Full table
In multivariable analyses (adjusted for variables in Table 1), perceived institutional barriers were the only predictors of reduced attempts to minimize TV with an adjusted odds ratio of 0.1 [95% confidence interval (CI): 0.03–0.6]. The omnibus test of model co-efficient was significant at P=0.01. The Hosmer-Lemeshow test could not reject the null hypothesis of model goodness-of-fit (P=0.47); thus, the model was presumed to have good fit.
Discussion & conclusions
To our knowledge, this study is the first to assess the reported practices and perspectives of anesthesiologists regarding LPV during OLV in North America & also the first to explore predictors of targeting low TVs. In this study, most anesthesiologists defined low PAP as the primary strategy of LPV during OLV and actively attempted to minimize it. There was a trend among respondents towards predominantly volume-controlled ventilation. Approximately 64% of responding anesthesiologists reported actively trying to minimize TV. In multivariable analyses, perceived institutional barriers were the only independent predictors of reduced attempts to minimize TV with an adjusted odds ratio of 0.1 (95% CI: 0.03–0.6). Thus, on self-report, anesthesiologists were significantly less likely to try minimizing TVs if they perceived institutional barriers to doing so. Thus, even though the majority of the anesthesiologists (76%) reported no institutional barriers to implementing low TVs, it seems that perceived institutional barriers may still be an important factor in knowledge translation. These analyses were adjusted for certain factors that may have influenced respondents’ likelihood of actively trying to minimize TV during OLV surgeries and included clinical experience (i.e., number of years in practice, percent of caseload spent doing OLV), extra training in the form of a cardiothoracic anesthesia fellowship and work as an ICU physician. Thus, when you control for these factors using statistical methods, it appears that clinical experience, extra cardiothoracic training or ICU work experience do not predict implementation of low TV. The main institutional barriers reported were a lack of a protocol to implement low TV during OLV and perceived surgeon/institutional bias against low TV during OLV. It is unclear from our study what specific surgeon or institutional bias the respondents perceived; perhaps the surgeons or institution were perceived to be biased against the risk of hypoxia or atelectasis during OLV surgery. Future mixed methods studies should explore the specific surgeon or institutional barriers against low TV perceived by anesthesiologists.
In a British study by Shelley et al., 40% of respondents reported ventilating to a target median TV of 6 cc/kg (interquartile range, 5–7) (18). This align with our finding that minimization of TV is not being universally applied by anesthesiologists overseeing OLV surgeries. Although Shelley et al.’s survey was not designed to investigate perspectives and practices regarding LPV, they comment that LPV strategies are becoming increasingly incorporated in the UK thoracic anesthesia practice (18). This was based on their findings regarding median TV targets as well reports of more permissive approaches to hypercapnia (18). A national Italian study surveyed 92 thoracic surgery centres in Italy and found that 51% of centres reported ventilating to a target TV of 4–6 cc/kg (19). They also found that 42.5% reported using mainly pressure-controlled ventilation. It is difficult to compare and contrast findings between these two studies and ours for several reasons. First, these two studies focussed solely on general practice patterns while the descriptive component of our study focussed specifically on intra-operative LPV during OLV surgeries. Secondly, these two studies were purely descriptive in nature and did not include an explanatory/inferential component as did ours.
This study has several limitations. First, this was a survey study of self-report data which has known risks of introducing bias. However, our objective of determining the perspectives of anesthesiologists could not have been accomplished in any other way. Furthermore, although self-report increases bias in determining practice patterns, we would expect self-report bias to be operating in a direction opposite to that observed in this study. Specifically, since the majority of respondents reported a belief that current evidence supported use of low TVs during OLV surgeries, we would expect self-report bias to operate in the direction of over-reporting use of LPV strategies and minimization of TV. Thus, the proportion of respondents who actively attempt to minimize TV may be actually much lower. Another weakness is the subtotal response rate, which is frequently inherent in this type of study design. There is a potential that availability of data from more respondents could have changed the magnitude and/or direction of effects reported in this study due to increased risks of nonresponse and selection biases (20,21). However, our response rate of 63% would be considered a very good response rate among survey studies, especially of health professionals (20,21). In fact our response rate was considerably higher than the response rates in the British (<30%) and Italian (<50%) studies (18,19). Finally, this study only touched briefly on the issue of PEEP as it relates to intra-operative LPV. A follow-up study will investigate its role within LPV strategies in current practices. This will be a national survey administered to the national association of anesthesiologists; it will utilize factor analysis of this current survey to further reduce the number of questions and thereby reduce non-response rate. Despite these limitations, this study has several strengths. It is novel and has the potential to inform future research in this area. Most importantly, however, the survey was designed, refined and administered using rigorous survey methodology.
Over the past decade, mounting evidence has shown that LPV during OLV surgeries is associated with a reduction in markers of lung injury (22-24). Most recently, a randomized controlled trial (RCT) with 100 patients demonstrated that intra-operative LPV during OLV resulted in significantly fewer cases of post-operative radiographic lung infiltration or atelectasis (2 vs. 10, P=0.03) and fewer cases of PaO2/FiO2 ratios of less than 300 (1 vs. 8, P=0.03) (13). The LPV protocol consisted of FiO2 =0.5, PEEP 5 cmH2O and TV of 6 cc/kg predicted body weight while the conventional ventilation protocol consisted of FiO2 =1.0, no PEEP and TV of 10 cc/kg predicted body weight. However, there was no difference in ALI rate despite the study being reportedly powered to detect such a difference. Currently, no strong evidence exists demonstrating a reduction of respiratory complications or mortality with use of intra-operative LPV strategies during OLV surgeries. Nevertheless, many experts in cardiothoracic anesthesia have called for adoption of LPV in this setting (7,10,15). RCTs with clinically-important outcomes (i.e., respiratory complications, mortality) are needed and, in fact, there are several RCTs currently or on the cusp of recruiting patients (25,26).
Ventilation management during OLV is a highly complex process that involves the interplay between TV, airway pressures, PEEP, patient lung function and patient body morphology. When low TVs are used patient-specific adjustments of PEEP, lung recruitments and adjustment of FiO2 are frequently necessary. When low TVs are used, atelectasis and hypoxia may result and can be harmful to patients. We had initially designed this 3-center survey in order to produce a relatively homogeneous reference standard in relation to responses from OLV (academic and non-academic) anesthesiologists across the country. Although there appeared to be homogeneity in minimization of PAP, there appeared to be heterogeneity in many of the other practices and perspectives. Although it is not clear what is driving this heterogeneity in practices and perspectives, we conjecture that it is at least partly due to the paucity of level I evidence showing benefit or harm for the use LPV during OLV surgeries. This may have resulted in many anesthesiologists adopting practices based on their particular training, clinical experience, influence from local experts as well as their own interpretation of the existing literature. Given the fact that the results of the national survey may not be available for several years, we felt it important to report this unanticipated heterogeneity in a timely fashion to the community of practitioners involved in OLV surgeries. This will afford the community an opportunity to engage in discussions and perhaps their own knowledge translation activities.
Our study suggests that there is a lack of universal definition of intra-operative LPV during OLV; moreover, it appears that many anesthesiologists prioritize minimizing PAP as opposed to TV. This lack of a consensus may render the results of future trials, regardless of how strong, difficult to interpret by end-users. Our study also suggests that institutional barriers may also subvert current and future knowledge translation activities. Such institutional barriers will hopefully be removed once good evidence from RCTs becomes available; nevertheless, our findings highlight the importance of identifying such barriers to implementation early on in the knowledge translation process.
Acknowledgements
The authors would like to acknowledge members of the ACCADEMY (Academy of Critical Care: Development, Evaluation and Methodology) Group for their help in the development and piloting of the survey.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics was approved for this survey study of health professionals.
Disclaimer: This work was presented as a podium presentation at the 49th Annual Meeting of the Society of Thoracic Surgeons in Los Angeles, California, USA (January 26–30, 2013).
References
- Ruffini E, Parola A, Papalia E, et al. Frequency and mortality of acute lung injury and acute respiratory distress syndrome after pulmonary resection for bronchogenic carcinoma. Eur J Cardiothorac Surg 2001;20:30-6; discussion 36-7. [Crossref] [PubMed]
- Kutlu CA, Williams EA, Evans TW, et al. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2000;69:376-80. [Crossref] [PubMed]
- Shapiro M, Swanson SJ, Wright CD, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2010;90:927-34; discussion 934-5. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637-45. [Crossref] [PubMed]
- Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. [Crossref] [PubMed]
- Peruzzi W. Lung protective strategy: many pieces of the puzzle. J Crit Care 2011;26:152-4. [Crossref] [PubMed]
- Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care 2005;11:82-6. [Crossref] [PubMed]
- Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol (1985) 2000;89:1645-55. [Crossref] [PubMed]
- Kilpatrick B, Slinger P. Lung protective strategies in anaesthesia. Br J Anaesth 2010;105 Suppl 1:i108-16. [Crossref] [PubMed]
- Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013;369:428-37. [Crossref] [PubMed]
- Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;308:1651-9. [Crossref] [PubMed]
- Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139:530-7. [Crossref] [PubMed]
- Licker M, Diaper J, Villiger Y, et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care 2009;13:R41. [Crossref] [PubMed]
- Sentürk M. New concepts of the management of one-lung ventilation. Curr Opin Anaesthesiol 2006;19:1-4. [Crossref] [PubMed]
- Dillman DA. Mail and Internet Surveys: The Tailored Design Method. 2nd edition. New York, NY: Wiley, 2007.
- Cancer Care Ontario. Thoracic Cancer Surgery Standards and Post-Surgical Mortality. Available online: http://www.csqi.on.ca/cms/One.aspx?portalId=126935&pageId=127980
- Shelley B, Macfie A, Kinsella J. Anesthesia for thoracic surgery: a survey of UK practice. J Cardiothorac Vasc Anesth 2011;25:1014-7. [Crossref] [PubMed]
- Della Rocca G, Langiano N, Baroselli A, et al. Survey of thoracic anesthetic practice in Italy. J Cardiothorac Vasc Anesth 2013;27:1321-9. [Crossref] [PubMed]
- Burns KE, Duffett M, Kho ME, et al. A guide for the design and conduct of self-administered surveys of clinicians. CMAJ 2008;179:245-52. [Crossref] [PubMed]
- Thoma A, Cornacchi SD, Farrokhyar F, et al. How to assess a survey in surgery. Can J Surg 2011;54:394-402. [Crossref] [PubMed]
- Yin K, Gribbin E, Emanuel S, et al. Histochemical alterations in one lung ventilation. J Surg Res 2007;137:16-20. [Crossref] [PubMed]
- Misthos P, Katsaragakis S, Theodorou D, et al. The degree of oxidative stress is associated with major adverse effects after lung resection: a prospective study. Eur J Cardiothorac Surg 2006;29:591-5. [Crossref] [PubMed]
- Funakoshi T, Ishibe Y, Okazaki N, et al. Effect of re-expansion after short-period lung collapse on pulmonary capillary permeability and pro-inflammatory cytokine gene expression in isolated rabbit lungs. Br J Anaesth 2004;92:558-63. [Crossref] [PubMed]
- Della Rocca G. Protective Versus Conventional Ventilation During Thoracic Surgery. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000. Available online: http://clinicaltrials.gov/show/NCT01504893
- Xue Z. A Trial to Evaluate the Impact of Lung-protective Intervention in Patients Undergoing Esophageal Cancer Surgery. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000. Available online: http://clinicaltrials.gov/show/NCT01194895


