What defines quality in small bowel capsule endoscopy
Introduction
Gastrointestinal endoscopy is an important tool for the diagnosis of alimentary tract diseases. Since the introduction of the fiber-optic endoscope, technological advances, complementary tools and endoscopic techniques, have been continuously evolving. Maintaining high endoscopic quality requires that procedures are ordered on the basis of appropriate indications, result in the recognition or exclusion of a clinically relevant diagnosis and facilitate appropriate, as well as effective therapeutic options, while minimizing patient risk (1).
In order to ensure healthcare quality, gastroenterological and endoscopic societies proposed and established quality indicators, also known as quality, or performance measures, for the majority of endoscopic procedures.
Quality indicators are parameters, used for the measurement of quality of care and services performance (2). They are categorized as structural, process and outcome measures (2) and depending on the time period related to the endoscopic procedure, they can be divided in pre-procedural, intra-procedural and post-procedural indicators (1). Their purpose is to improve care and processes, ensure competency and elucidate areas that need further research, without however being a direct measure of quality (2).
Although the available literature describing endoscopic procedure quality indicators is growing (1,3-10), there is a paucity of publications regarding small bowel capsule endoscopy (SBCE) quality indicators, with the only available report published by the Korean Gut Image Study Group (KGISG), under the approval of the Korean Society of Gastrointestinal Endoscopy (KSGE) (11). Hereby, we attempt to identify and describe a number of capsule endoscopy quality indicators regarding process measures, after a comprehensive review of the literature
Methodology
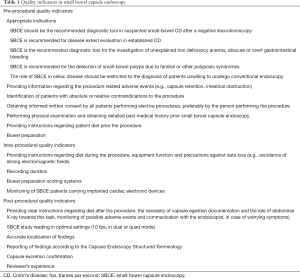
A thorough review of the literature of studies published after 2000 was performed using PubMed, in order to identify articles that describe factors affecting quality in SBCE. The search for SBCE studies was performed using the keywords: “small bowel capsule endoscopy”, “SBCE”, “wireless capsule endoscopy”, “capsule endoscopy” and “video capsule”. Furthermore, publications from gastroenterological and endoscopic societies, which describe quality indicators in endoscopic procedures and relevant recommendations, were also reviewed. Of note, the vast majority of capsule endoscopy studies are based on PillCam (Given Imaging, Yokneam, Israel) small bowel capsule endoscopes. As in the case of other endoscopic procedures, SBCE indicators will be divided in pre-procedure, intra-procedure and post-procedure quality indicators. A summary of the described quality indicators can be found in Table 1.
Full table
Preprocedure quality indicators
In this section will be described quality indicators that apply to the time period prior to the capsule endoscope ingestion.
Appropriate indications
A correctly indicated procedure results in relevant diagnosis, is able to guide therapeutic, as well as management planning and results in favorable patient outcomes.
SBCE major indications are: (I) suspected small-bowel Crohn’s disease diagnosis after a negative ileocolonoscopy; (II) established Crohn’s disease extent evaluation; (III) investigation of unexplained iron deficiency anemia, as well as obscure or overt gastrointestinal bleeding; (IV) detection of small-bowel polyps due to familial or other polyposis syndromes and (V) celiac disease diagnosis in patients unwilling to undergo conventional endoscopy (12).
SBCE, should be the first-line diagnostic tool for the investigation of suspected small bowel bleeding, due to the reliability and reproducibility of its findings (13,14). When performed during overt gastrointestinal bleeding, the diagnostic yield of SBCE is increased and is demonstrated to exceed 90% (15,16). The diagnostic yield decreases in cases of occult or previous gastrointestinal bleeding (15), thus SBCE should be performed as close as possible and no longer than 2 weeks from the bleeding episode, in order to maximize the possibility of lesion identification (12).
Suspected Crohn’s disease patients scheduled for capsule endoscopy, should be carefully selected (17), as the symptoms of abdominal pain and diarrhea, without other accompanying signs, for example extra-intestinal manifestations or elevated inflammatory markers, result in poor diagnostic yield (18-21). Of note, SBCE is not purposed to substitute esophagogastroduodenoscopy (EGD) in the diagnosis of celiac disease and should only be reserved for patients unwilling to undergo conventional endoscopy (12).
Providing information regarding procedure related adverse events
The introduction of SBCE in 2000 (22), provided a non-invasive, well tolerated method for the visualization of the small bowel. However, patients should be aware that its use is not devoid of complications.
In general, the adverse event rate is low, estimated at 1–3% (12,23,24). Capsule endoscope retention is the most frequent complication, which may result in intestinal obstruction and perforation. This risk is minimal in healthy individual whereas in patients with established Crohn’s disease is estimated to be 2.5%, despite initial reports demonstrating a risk of 13% (24-29). Major factors for capsule retention are non-steroidal anti-inflammatory drugs enteropathy, abdominal surgery, intestinal ischemia, volvulus and radiotherapy of the abdominal or pelvic area (30-33). The successful gastrointestinal patency assessment, with the aid of cross-sectional imaging modalities or the patency capsule, allows the safe administration of capsule endoscopes to high risk patients (29,34). However, its administration is contraindicated in patients with a high likelihood of gastrointestinal obstruction, namely, known intestinal stenoses, fistulas (35) or large masses detected by cross-sectional studies (36).
Identification of patients with absolute or relative contraindications to the procedure
Capsule endoscope manufacturers and the United States Food and Drug Administration (FDA), do not recommend the use of capsule endoscopes in patients with pacemakers, cardioverters or left heart assist devices (35), despite the available evidence of the safety of this practice (37,38). Moreover, they also restrict its use in pregnant women due to the lack of safety data in this population, except in cases of emergency (39). Finally, capsule endoscope aspiration is a rare complication affecting patients with swallowing disabilities (35), thus, assisted endoscopic administration should be considered in this patient group (39).
Obtaining informed consent
An informed consent (40,41) has ethical and legal implications and it should preferably be obtained by the person performing the procedure. Information about the type of the procedure, indication, benefit, procedure complications, alternatives and prognosis if the examination is denied, should be given in the patient’s own language and in the case of elective examinations, it should be written. This practice facilitates patients’ choice in proceeding, or not, with SBCE and allows them to understand, as well as to ask details about the examination. Due to the non-invasive character of capsule endoscopy, the adverse event risks from its use should be mentioned clearly, without dissuading the patient from undergoing the examination.
Performing physical examination and obtaining past medical history
As in all endoscopic procedures, all patients should undergo physical examination and a detailed past medical history should be obtained prior SBCE. Accessing patient’s medical history is crucial, as (I) it allows the physician to assess the indication and benefit from the procedure; (II) it reveals comorbidities which prohibit the use of capsule endoscopes or may result in adverse events (for example implanted electrical devices and symptoms highly suggestive of intestinal obstruction) and (III) it enables the identification of patients who could benefit from endoscopic assisted capsule endoscope administration (39), for example patients with swallowing difficulties, gastroparesis, gastroenteroanastomosis, motility difficulties, previously failed capsule test, as well as medications and health conditions that affect intestinal transit. Furthermore, it facilitates the evaluation of bowel preparation level, allowing a reschedule in case there is high suspicion that the mucosa will not be adequately visualized.
Providing instructions regarding patient diet prior the procedure
The day before the procedure, patients should be requested to continue with a liquid diet after lunch, in order to have a successful capsule endoscopy examination. Ten hours before the procedure, they should be requested to stop the intake of liquids or solids and they should be reminded that they are not allowed to take any medication 2 hours prior capsule ingestion (42).
Bowel preparation
Intestinal chyme, bile or bubbles may compromise small bowel mucosa visualization (43) and the endoscopist’s ability to identify and interpret findings. Unlike the established importance of bowel preparation in colonoscopy (6), the role of intestinal preparation in the diagnostic yield of SBCE remains controversial. Various preparation regimens have been proposed, however the administration of 2 liters of polyethylene glycol the evening prior the procedure, remains the recommended preparation regimen (44). A significant number of meta-analysis (45-50) attempted to shed light in the role of purgative preparation and SBCE outcomes. Despite the fact that the included studies suffered from significant heterogeneity, in their majority they agreed that purgative preparation improved visual quality, without affecting diagnostic yield and completion rate. Nevertheless, a recent meta-analysis (51) questions the role of purgative bowel preparation, both in improving mucosa visibility and increasing diagnostic yield. Since strong evidence regarding the role of intestinal preparation in small bowel endoscopy is still unavailable, large multicenter randomized-controlled trials are needed to evaluate the need, appropriate dose and time of purgative preparation prior capsule endoscopy.
Intra-procedure quality indicators.
Unlike any other endoscope, the capsule endoscope moves passively in the gastrointestinal tract, with the help of intestinal peristalsis and gravity, practically allowing no interventions to be applied during recording. Below we describe a number of quality measures that apply to the period between capsule ingestion and recording completion.
Providing intra-procedure instructions
According to the small bowel capsule endoscope manufacturer recommendations (52,53), patients should be requested to drink colorless liquids and have a light snack, 2 and 4 hours after capsule ingestion, respectively. Every 15 minutes during recording, patients should check that the blue flashing light of the recorder is blinking twice per second and in case it stops or changes color, the endoscopist should be contacted immediately. Every patient should be supplied with a capsule endoscopy event form, which will be returned to the endoscopist after the completion of the recording time, where they can note the time of events such as eating, drinking or changes in their activity. After capsule ingestion, patients should avoid getting close to strong electromagnetic fields such as magnetic resonance imaging (MRI) devices (35), or be exposed on direct bright sunlight until the procedure completion.
Recording duration
Older small bowel capsule endoscopes (M2A, SB2) offered 8 hours of operating time, resulting in a 20–30% procedure incompletion rate (54,55). If available, the use of newer capsule endoscopes with longer battery life should be preferred (e.g., the 3rd generation PillCam™, SB3, Given Imaging, Yokneam, Israel), as longer recording duration is demonstrated to increase study completion, although without a significant benefit in diagnostic yield (56-58).
Wireless capsule endoscope systems include handheld viewers (e.g., Rapid® Real-time, Given Imaging; Real Time Viewer, Olympus, America; Miro-View™ Express, Intro Medic), enabling the endoscopist to view in real-time the images of the recording procedure and terminate the examination if there is evidence that it has reached the cecum, or alternatively, prolong the procedure if necessary (59). Furthermore, it allows the endoscopist to identify stasis of the capsule in the gastric area, allowing the timely administration of prokinetics in an effort to propel the capsule to the small intestine (60). However, prokinetics can cause rapid intestinal passage, which may result in diminished diagnostic yield, so their use should be avoided, unless necessary (61,62).
Bowel preparation scoring systems
Numerous operator or computer dependent, quantitative and qualitative scoring systems were developed, for the objective assessment of intestinal preparation in capsule endoscopy, in an attempt to evaluate the reliability of findings and diagnostic accuracy.
However, operator dependent scoring systems demonstrate varying inter-observer and intra-observer agreement, can be time consuming, complicated and difficult to be applied in the everyday clinical practice (63). Contrary, computer dependent scales may prove a promising solution for the future of SBCE, as they are shown to overcome the aforementioned limitations (63). Nevertheless, their value in SBCE is under investigation.
Monitoring of SBCE patients carrying implanted cardiac electronic devices
The small bowel capsule endoscope communicates with its sensor through digital radiofrequencies. This type of communication is vulnerable to strong electromagnetic field interferences, for example MRI devices (35), which may result in signal interruption and loss of data transfer. Contrary, there is no proven interference with everyday electric appliances (e.g., cell phones, computers, home electric appliances etc.), so their use is not prohibited during recording.
As mentioned above, capsule endoscope manufacturers and the FDA are against the use of capsule endoscopy in patients carrying implanted cardiac devices (e.g., pacemakers, cardioverter defibrillators, and left heart assist devices) (64), for fear of possible interruptions that may result in their malfunctioning. Nevertheless, there is supporting evidence that the application of wireless capsule endoscopy in this patient group is relatively safe, with only a limited number of publications demonstrating malfunction of the implanted cardiac devices (37,38). Due to the above observations, patients with implantable cardiac devices undergoing capsule endoscopy should be monitored closely until capsule egestion, before the above recommendation is subjected to re-evaluation (64,65).
Post-procedure quality indicators
Below we present a number of quality measures that apply to the time period after recording time completion.
Providing post-procedure instructions
Patients should return to the doctor’s office at scheduled time to have their equipment removed. They should not be allowed to remove the equipment, unless they are instructed to do so and without damaging the equipment. After the examination completion, patients may return to their normal diet. During the post-procedure period they should be requested to document capsule egestion and in case of uncertainty, they should have an abdominal X-ray. Furthermore, patients should contact their physician in case that symptoms suggestive of intestinal obstruction occur (abdominal pain, nausea, vomiting).
SBCE study reading in optimal settings
A small bowel capsule endoscope video contains a combination of approximately 50,000 images. Initial studies estimated the average reviewing time for a capsule endoscopy study to be over 40 minutes (66-69). This finding was indicative that technological advents able to reduce reading duration, without affecting detection ability, were necessary. Workstation software innovations resulted in a significant reduction of reading time (70,71), as they allow the endoscopist to review every single image of the created video (manual function), or to review a video where the repetitive images have been excluded (automatic function). Endoscopists should be aware that delayed regional transit may indicate underlying pathology (72), a finding that warrants further investigation that may be missed when automatic function is used.
Moreover, capsule endoscopy software offers a variety of viewing modes in different frame rates. The chosen speed (frame rate) and number of simultaneous frames shown in the workstation monitor (single, dual, and quad view mode), affect the reviewer’s lesion detection ability and finding interpretation (71).
The optimal capsule endoscopy reviewing settings are 10 frames per second (fps) in dual or quad view modes. Lower fps result in more lesions detected, at the cost of longer reading duration (73,74). Dual or quad view modes allow longer reviewing time per image compared to single view, resulting in higher efficiency and detection rate compared to single view (73).
Another utility of the Given Imaging capsule endoscope software allowing reading time reduction, is the quick view mode. In quick view mode, with the help of a specific algorithm, 10% of the recorded images are selected, with a sampling rate between 5–80% (75) resulting in a shortened version of the created video. Nevertheless, image selection may result in missed findings, thus the use of this function is recommended when extended lesions are expected, for example known or highly suspected Crohn’s disease and celiac disease (70,75-79).
Accurate localization of findings
Accurate localization of findings and landmark setting influences further management and therapeutic planning. Currently the only feasible method to localize findings is by dividing the small bowel in three segments, proximal, mid and distal parts, based on transit times until more precise localization technology will be available (65).
Reporting of findings according to the capsule endoscopy structured terminology (CEST)
The introduction of the Given Imaging small bowel capsule endoscope, generated the need for an official lexicon for the description of its indications and study findings. This lexicon, namely the CEST (80), was the result of a consensus between experts in the field of capsule endoscopy and it was influenced by the minimal standard terminology for digestive endoscopy (81). CEST should be used as the standard lexicon for capsule endoscopy procedures as it allows the endoscopist to describe the vast majority of findings and improves report quality (82).
Capsule excretion confirmation
Capsule endoscope retention may result in the life-threatening complications of intestinal obstruction and perforation, making necessary the evidenced excretion of the capsule endoscope to successful establish the completion of the examination. Usually intestinal transit requires 6 hours (83) and the capsule endoscope requires 24–48 hours (15,84) to be excreted from the human body. In the case that the patient is unable to identify the egested capsule and there is fear of a complication, then an abdominal X-ray should be ordered to exclude or confirm its presence in the gastrointestinal tract.
On the other hand, if the interpretation of the recorded video shows a passage of the capsule endoscope to the large bowel and the patient has undergone a previous colonoscopy without evidence of a stricture, an uneventful egestion of the capsule endoscope from the body can be expected without any further evaluation or follow-up.
Reviewer’s experience
A capsule endoscopy trainee should complete a minimum number of capsule endoscopy studies to reach a sufficient level of competency. Although this number is estimated between 10 to 20 capsule endoscopy studies (85,86), the capsule endoscopy examination accuracy increases along with the trainee’s learning curve and reaches a plateau after the first 100 studies (87). Of note, readers with experience in endoscopic image interpretation are preferred (86,88), although previous endoscopic experience was not shown to be associated with capsule endoscope competence (85).
Conclusions
This paper describes quality indicators related to SBCE, in order to ensure high endoscopic quality and eliminate differences in clinical practice. Although the majority of proposed indicators are based on available evidence, the lack of randomized controlled trials elucidates the need of further research who will measure their influence in health outcomes and capsule endoscopy competence, to support the recommended measures.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rizk MK, Sawhney MS, Cohen J, et al. Quality indicators common to all GI endoscopic procedures. Am J Gastroenterol 2015;110:48-59. [Crossref] [PubMed]
- Mainz J. Defining and classifying clinical indicators for quality improvement. Int J Qual Health Care 2003;15:523-30. [Crossref] [PubMed]
- Bisschops R, Areia M, Coron E, et al. Performance measures for upper gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy quality improvement initiative. United European Gastroenterol J 2016;4:629-56. [Crossref] [PubMed]
- Kaminski MF, Thomas-Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2017;49:378-97. [Crossref] [PubMed]
- Park WG, Shaheen NJ, Cohen J, et al. Quality indicators for EGD. Gastrointest Endosc 2015;81:17-30. [Crossref] [PubMed]
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol 2015;110:72-90. [Crossref] [PubMed]
- Adler DG, Lieb JG 2nd, Cohen J, et al. Quality indicators for ERCP. Am J Gastroenterol 2015;110:91-101. [Crossref] [PubMed]
- Wani S, Wallace MB, Cohen J, et al. Quality indicators for EUS. Am J Gastroenterol 2015;110:102-13. [Crossref] [PubMed]
- Beg S, Ragunath K, Wyman A, et al. Quality standards in upper gastrointestinal endoscopy: a position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut 2017;66:1886-99. [Crossref] [PubMed]
- Rees CJ, Thomas Gibson S, Rutter MD, et al. UK key performance indicators and quality assurance standards for colonoscopy. Gut 2016;65:1923-9. [Crossref] [PubMed]
- Shim KN, Jeon SR, Jang HJ, et al. Quality Indicators for Small Bowel Capsule Endoscopy. Clin Endosc 2017;50:148-60. [Crossref] [PubMed]
- Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015;47:352-76. [Crossref] [PubMed]
- Christodoulou DK, Haber G, Beejay U, et al. Reproducibility of wireless capsule endoscopy in the investigation of chronic obscure gastrointestinal bleeding. Can J Gastroenterol 2007;21:707-14. [Crossref] [PubMed]
- Tang SJ, Christodoulou D, Zanati S, et al. Wireless capsule endoscopy for obscure gastrointestinal bleeding: a single-centre, one-year experience. Can J Gastroenterol 2004;18:559-65. [Crossref] [PubMed]
- Pennazio M, Santucci R, Rondonotti E, et al. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology 2004;126:643-53. [Crossref] [PubMed]
- Carey EJ, Leighton JA, Heigh RI, et al. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol 2007;102:89-95. [Crossref] [PubMed]
- Mergener K, Ponchon T, Gralnek I, et al. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy 2007;39:895-909. [Crossref] [PubMed]
- May A, Manner H, Schneider M, et al. Prospective multicenter trial of capsule endoscopy in patients with chronic abdominal pain, diarrhea and other signs and symptoms (CEDAP-Plus Study). Endoscopy 2007;39:606-12. [Crossref] [PubMed]
- Bardan E, Nadler M, Chowers Y, et al. Capsule endoscopy for the evaluation of patients with chronic abdominal pain. Endoscopy 2003;35:688-9. [Crossref] [PubMed]
- Katsinelos P, Fasoulas K, Beltsis A, et al. Diagnostic yield and clinical impact of wireless capsule endoscopy in patients with chronic abdominal pain with or without diarrhea: a Greek multicenter study. Eur J Intern Med 2011;22:e63-6. [Crossref] [PubMed]
- Mitselos IV, Christodoulou DK, Katsanos KH, et al. The role of small bowel capsule endoscopy and ileocolonoscopy in patients with nonspecific but suggestive symptoms of Crohn's disease. Eur J Gastroenterol Hepatol 2016;28:882-9. [Crossref] [PubMed]
- Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature 2000;405:417. [Crossref] [PubMed]
- Koulaouzidis A, Rondonotti E, Karargyris A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J Gastroenterol 2013;19:3726-46. [Crossref] [PubMed]
- Rezapour M, Amadi C, Gerson LB, et al. Retention associated with video capsule endoscopy: systematic review and meta-analysis. Gastrointest Endosc 2017;85:1157-68.e2. [Crossref] [PubMed]
- Cheifetz AS, Kornbluth AA, Legnani P, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn's disease. Am J Gastroenterol 2006;101:2218-22. [Crossref] [PubMed]
- Mow WS, Lo SK, Targan SR, et al. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin Gastroenterol Hepatol 2004;2:31-40. [Crossref] [PubMed]
- Leighton JA, Gralnek IM, Cohen SA, et al. Capsule endoscopy is superior to small-bowel follow-through and equivalent to ileocolonoscopy in suspected Crohn's disease. Clin Gastroenterol Hepatol 2014;12:609-15. [Crossref] [PubMed]
- Mehdizadeh S, Chen GC, Barkodar L, et al. Capsule endoscopy in patients with Crohn's disease: diagnostic yield and safety. Gastrointest Endosc 2010;71:121-7. [Crossref] [PubMed]
- Nemeth A, Kopylov U, Koulaouzidis A, et al. Use of patency capsule in patients with established Crohn's disease. Endoscopy 2016;48:373-9. [PubMed]
- Cave D, Legnani P, de Franchis R, et al. ICCE consensus for capsule retention. Endoscopy 2005;37:1065-7. [Crossref] [PubMed]
- Goenka MK, Majumder S, Goenka U. Capsule endoscopy: Present status and future expectation. World J Gastroenterol 2014;20:10024-37. [Crossref] [PubMed]
- Yadav A, Heigh RI, Hara AK, et al. Performance of the patency capsule compared with nonenteroclysis radiologic examinations in patients with known or suspected intestinal strictures. Gastrointest Endosc 2011;74:834-9. [Crossref] [PubMed]
- Bjarnason I, Price AB, Zanelli G, et al. Clinicopathological features of nonsteroidal antiinflammatory drug-induced small intestinal strictures. Gastroenterology 1988;94:1070-4. [Crossref] [PubMed]
- Rommele C, Brueckner J, Messmann H, et al. Clinical Experience with the PillCam Patency Capsule prior to Video Capsule Endoscopy: A Real-World Experience. Gastroenterol Res Pract 2016;2016. [Crossref] [PubMed]
- PillCam™ SB 3 Indications. Available online: http://www.medtronic.com/covidien/en-us/products/capsule-endoscopy/pillcam-sb-3-system/indications.html
- Saito K, Nakagawa T, Koseki H, et al. Retention of the cellophane wall of a patency capsule by intestinal stenosis: a report of three cases. Clin J Gastroenterol 2016;9:365-8. [Crossref] [PubMed]
- Dubner S, Dubner Y, Rubio H, et al. Electromagnetic interference from wireless video-capsule endoscopy on implantable cardioverter-defibrillators. Pacing Clin Electrophysiol 2007;30:472-5. [Crossref] [PubMed]
- Harris LA, Hansel SL, Rajan E, et al. Capsule Endoscopy in Patients with Implantable Electromedical Devices is Safe. Gastroenterol Res Pract 2013;2013. [Crossref] [PubMed]
- Bandorski D, Kurniawan N, Baltes P, et al. Contraindications for video capsule endoscopy. World J Gastroenterol 2016;22:9898-908. [Crossref] [PubMed]
- Everett SM, Griffiths H, Nandasoma U, et al. Guideline for obtaining valid consent for gastrointestinal endoscopy procedures. Gut 2016;65:1585-601. [Crossref] [PubMed]
- Standards of Practice C, Zuckerman MJ, Shen B, et al. Informed consent for GI endoscopy. Gastrointest Endosc 2007;66:213-8. [Crossref] [PubMed]
- Committee AT, Wang A, Banerjee S, et al. Wireless capsule endoscopy. Gastrointest Endosc 2013;78:805-15. [Crossref] [PubMed]
- Song HJ, Moon JS, Shim KN. Optimal Bowel Preparation for Video Capsule Endoscopy. Gastroenterol Res Pract 2016;2016. [Crossref] [PubMed]
- Rey JF, Gay G, Kruse A, et al. European Society of Gastrointestinal Endoscopy guideline for video capsule endoscopy. Endoscopy 2004;36:656-8. [Crossref] [PubMed]
- Niv Y. Efficiency of bowel preparation for capsule endoscopy examination: a meta-analysis. World J Gastroenterol 2008;14:1313-7. [Crossref] [PubMed]
- Rokkas T, Papaxoinis K, Triantafyllou K, et al. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy?: A meta-analysis. Am J Gastroenterol 2009;104:219-27. [Crossref] [PubMed]
- Belsey J, Crosta C, Epstein O, et al. Meta-analysis: efficacy of small bowel preparation for small bowel video capsule endoscopy. Curr Med Res Opin 2012;28:1883-90. [Crossref] [PubMed]
- Kotwal VS, Attar BM, Gupta S, et al. Should bowel preparation, antifoaming agents, or prokinetics be used before video capsule endoscopy? A systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2014;26:137-45. [Crossref] [PubMed]
- Wu S, Gao YJ, Ge ZZ. Optimal use of polyethylene glycol for preparation of small bowel video capsule endoscopy: a network meta-analysis. Curr Med Res Opin 2017;33:1149-54. [Crossref] [PubMed]
- Yung DE, Rondonotti E, Sykes C, et al. Systematic review and meta-analysis: is bowel preparation still necessary in small bowel capsule endoscopy? Expert Rev Gastroenterol Hepatol 2017;11:979-93. [Crossref] [PubMed]
- Gkolfakis P, Tziatzios G, Dimitriadis GD, et al. Meta-analysis of randomized controlled trials challenging the usefulness of purgative preparation before small-bowel video capsule endoscopy. Endoscopy 2018;50:671-83. [Crossref] [PubMed]
- Patient Instructions for PillCam® Small Bowel Capsule Endoscopy with the Sensor Belt. Available online: http://www.medtronic.com/content/dam/covidien/library/us/en/product/diagnostic-testing/pillcam-sb-sensor-belt-patient-instructions.pdf
- Patient Instructions for PillCam® Small Bowel Capsule Endoscopy with the Sensor Array. Available online: http://www.medtronic.com/content/dam/covidien/library/us/en/product/diagnostic-testing/pillcam-sb-sensor-array-patient-instructions.pdf
- Hoog CM, Bark LA, Arkani J, et al. Capsule retentions and incomplete capsule endoscopy examinations: an analysis of 2300 examinations. Gastroenterol Res Pract 2012;2012. [Crossref] [PubMed]
- Westerhof J, Weersma RK, Koornstra JJ. Risk factors for incomplete small-bowel capsule endoscopy. Gastrointest Endosc 2009;69:74-80. [Crossref] [PubMed]
- Rahman M, Akerman S, DeVito B, et al. Comparison of the diagnostic yield and outcomes between standard 8 h capsule endoscopy and the new 12 h capsule endoscopy for investigating small bowel pathology. World J Gastroenterol 2015;21:5542-7. [Crossref] [PubMed]
- Ou G, Shahidi N, Galorport C, et al. Effect of longer battery life on small bowel capsule endoscopy. World J Gastroenterol 2015;21:2677-82. [Crossref] [PubMed]
- Pioche M, Gaudin JL, Filoche B, et al. Prospective, randomized comparison of two small-bowel capsule endoscopy systems in patients with obscure GI bleeding. Gastrointest Endosc 2011;73:1181-8. [Crossref] [PubMed]
- Yague AS, Zabal JMR, Méndez-Sánchez IM, et al. Protocol Based On the Rapid RT Real Time Viewer to Prevent Incomplete Capsule Endoscopy Studies. Gastrointestinal Endoscopy 2009;69:AB203. [Crossref]
- Hosono K, Endo H, Sakai E, et al. Optimal approach for small bowel capsule endoscopy using polyethylene glycol and metoclopramide with the assistance of a real-time viewer. Digestion 2011;84:119-25. [Crossref] [PubMed]
- Fireman Z, Paz D, Kopelman Y. Capsule endoscopy: improving transit time and image view. World J Gastroenterol 2005;11:5863-6. [Crossref] [PubMed]
- Buscaglia JM, Kapoor S, Clarke JO, et al. Enhanced diagnostic yield with prolonged small bowel transit time during capsule endoscopy. Int J Med Sci 2008;5:303-8. [Crossref] [PubMed]
- Ponte A, Pinho R, Rodrigues A, et al. Review of small-bowel cleansing scales in capsule endoscopy: A panoply of choices. World J Gastrointest Endosc 2016;8:600-9. [Crossref] [PubMed]
- Bandorski D, Holtgen R, Stunder D, et al. Capsule endoscopy in patients with cardiac pacemakers, implantable cardioverter defibrillators and left heart assist devices. Ann Gastroenterol 2014;27:3-8. [PubMed]
- Van de Bruaene C, De Looze D, Hindryckx P. Small bowel capsule endoscopy: Where are we after almost 15 years of use? World J Gastrointest Endosc 2015;7:13-36. [Crossref] [PubMed]
- Sidhu R, Sanders DS, Morris AJ, et al. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut 2008;57:125-36. [Crossref] [PubMed]
- Levinthal GN, Burke CA, Santisi JM. The accuracy of an endoscopy nurse in interpreting capsule endoscopy. Am J Gastroenterol 2003;98:2669-71. [Crossref] [PubMed]
- Niv Y, Niv G. Capsule endoscopy examination--preliminary review by a nurse. Dig Dis Sci 2005;50:2121-4. [Crossref] [PubMed]
- Pennazio M. Capsule endoscopy: where are we after 6 years of clinical use? Dig Liver Dis 2006;38:867-78. [Crossref] [PubMed]
- Westerhof J, Koornstra JJ, Weersma RK. Can we reduce capsule endoscopy reading times? Gastrointest Endosc 2009;69:497-502. [Crossref] [PubMed]
- Gunther U, Daum S, Zeitz M, et al. Capsule endoscopy: comparison of two different reading modes. Int J Colorectal Dis 2012;27:521-5. [Crossref] [PubMed]
- Tang SJ, Zanati S, Dubcenco E, et al. Capsule endoscopy regional transit abnormality: a sign of underlying small bowel pathology. Gastrointest Endosc 2003;58:598-602. [PubMed]
- Nakamura M, Murino A, O'Rourke A, et al. A critical analysis of the effect of view mode and frame rate on reading time and lesion detection during capsule endoscopy. Dig Dis Sci 2015;60:1743-7. [Crossref] [PubMed]
- Zheng Y, Hawkins L, Wolff J, et al. Detection of lesions during capsule endoscopy: physician performance is disappointing. Am J Gastroenterol 2012;107:554-60. [Crossref] [PubMed]
- Halling ML, Nathan T, Kjeldsen J, et al. High sensitivity of quick view capsule endoscopy for detection of small bowel Crohn's disease. J Gastroenterol Hepatol 2014;29:992-6. [Crossref] [PubMed]
- Shiotani A, Honda K, Kawakami M, et al. Analysis of small-bowel capsule endoscopy reading by using Quickview mode: training assistants for reading may produce a high diagnostic yield and save time for physicians. J Clin Gastroenterol 2012;46:e92-5. [Crossref] [PubMed]
- Koulaouzidis A, Smirnidis A, Douglas S, et al. QuickView in small-bowel capsule endoscopy is useful in certain clinical settings, but QuickView with Blue Mode is of no additional benefit. Eur J Gastroenterol Hepatol 2012;24:1099-104. [Crossref] [PubMed]
- Kyriakos N, Karagiannis S, Galanis P, et al. Evaluation of four time-saving methods of reading capsule endoscopy videos. Eur J Gastroenterol Hepatol 2012;24:1276-80. [PubMed]
- Shiotani A, Honda K, Kawakami M, et al. Evaluation of RAPID((R)) 5 Access software for examination of capsule endoscopies and reading of the capsule by an endoscopy nurse. J Gastroenterol 2011;46:138-42. [Crossref] [PubMed]
- Korman LY, Delvaux M, Gay G, et al. Capsule endoscopy structured terminology (CEST): proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy 2005;37:951-9. [Crossref] [PubMed]
- Delvaux M, Crespi M, Armengol-Miro JR, et al. Minimal standard terminology for digestive endoscopy: results of prospective testing and validation in the GASTER project. Endoscopy 2000;32:345-55. [Crossref] [PubMed]
- Delvaux M, Friedman S, Keuchel M, et al. Structured terminology for capsule endoscopy: results of retrospective testing and validation in 766 small-bowel investigations. Endoscopy 2005;37:945-50. [Crossref] [PubMed]
- Kim ER, Rhee PL. How to interpret a functional or motility test - colon transit study. J Neurogastroenterol Motil 2012;18:94-9. [Crossref] [PubMed]
- Delvaux M, Gerard G. Capsule endoscopy in 2005: facts and perspectives. Best Pract Res Clin Gastroenterol 2006;20:23-39. [Crossref] [PubMed]
- Rajan E, Iyer PG, Oxentenko AS, et al. Training in small-bowel capsule endoscopy: assessing and defining competency. Gastrointest Endosc 2013;78:617-22. [Crossref] [PubMed]
- Korean Gut Image Study Group, Lim YJ, Joo YS, et al. Learning curve of capsule endoscopy. Clin Endosc 2013;46:633-6. [Crossref] [PubMed]
- McAlindon ME, Ching HL, Yung D, et al. Capsule endoscopy of the small bowel. Ann Transl Med 2016;4:369. [Crossref] [PubMed]
- Velayos Jimenez B, Alcaide Suarez N, Gonzalez Redondo G, et al. Impact of the endoscopist's experience on the negative predictive value of capsule endoscopy. Gastroenterol Hepatol 2017;40:10-5. [PubMed]


