Advances on systemic treatment for lung neuroendocrine neoplasms
Introduction
Neuroendocrine tumors (NETs) encompass a group of neoplasms that are derived from the diffuse endocrine system and have the ability to synthesize and secrete compounds that, when biologically active, can give rise to distinct clinical syndromes. As neuroendocrine cells are scattered throughout the body, NETs can occur in virtually any organ. While the great majority arises in the gastrointestinal tract, lungs are the second most common site of involvement.
It is estimated that approximately 20–25% of all primary lung neoplasms are NETs and represent a spectrum of tumors arising from neuroendocrine cells of the bronchopulmonary epithelium (1). According to the latest WHO classification, lung NETs are categorized into the following four subtypes—typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine lung carcinoma (LCNELC) and small cell lung carcinoma (SCLC) (2). SCLC is a poorly differentiated (high grade) NET with extremely adverse prognosis and accounts for the majority (80%) of lung NETs (1). Because of its higher incidence and aggressive biology, the management of SCLC is reviewed in a different article in this issue.
By contrast, lung carcinoids (LCs) and LCNELC are less frequent. LCs are well-differentiated NETs and account for 8% of lung NETs and for 1–2% of all lung tumors (3). They are divided into the low-grade TCs with minimal mitotic activity (<2 mitoses per 2-mm2 field) without any necrosis, and the intermediate-grade ACs with more mitoses (2–10 per 2-mm2 field) and presence of focal necrosis. On the other hand, LCNELC is a poorly differentiated lung NET or NEC with morphological appearance resembling non-small cell lung cancer (NSCLC) but biological similarities with SCLC. LCNELC accounts for approximately 12% of lung NETs and is found in 2.1–3.5% of surgically resected bronchopulmonary neoplasms (4). Due to the rarity of LCs and LCNELC, there is lack of prospective studies, and relative paucity of strong evidence regarding their appropriate management, especially in the advanced disease setting. Most of the relevant data is obtained from either clinical trials involving different types of NETs with under-representation of lung NETs, or based on retrospectively collected data. The aim of this review is to provide relevant evidence, summarize current recommendations and present the therapeutic advances for control of tumor growth, in this field of rare tumors.
LCs
Even though LCs have a generally more indolent behavior compared with other primary lung tumors, they exhibit in some cases metastatic potential, necessitating appropriate treatment to achieve cure or long-term survival. The main determinants of treatment plan are tumors’ histologic subtype and extent of the disease. These two parameters are closely correlated, as the relatively more aggressive ACs has a greater propensity of nodal involvement and distant spread compared to the relatively more indolent TCs. Different case series have shown that the incidence of lymphatic and hematogenous metastases in ACs range from 20% to 60% and 16% to 21%, respectively, while in TCs the reported rates of metastases are substantially lower (9–12% and 1.5–3% respectively) (5).
Another critical aspect of LCs that affects treatment decisions is functionality, which means their capability of causing secretory syndromes that are named paraneoplastic when the secretory component is not derived from the expected tissue of origin. However, the majority are non-secretory and usually present similar to other lung cancers (cough, hemoptysis, fever). Paraneoplastic syndromes develop in approximately 10–15% of cases, the most common being carcinoid syndrome or Cushing’s syndrome secondary to ectopic serotonin or adrenocorticotropic hormone (ACTH) hypersecretion by tumor cells (6).
With regard to the staging of LCs, the use of the American Joint Committee on Cancer (AJCC) TNM staging system for lung, is adopted by many researchers, since, when applied, is associated with significant differences in survival (7). For the purposes of this review, we divide LCs in resectable and advanced/metastatic disease.
Resectable disease
LCs are mainly localized tumors and surgical removal remains the cornerstone of their treatment. With regard to the primary tumor, there are several surgical approaches, that are grossly divided into complete anatomic resection (lobectomy, bilobectomy, and pneumonectomy), sublobar resection (segmentectomy, wedge resection) and lung parenchyma-sparing surgery (bronchial sleeve resection, sleeve lobectomy). The choice of the most appropriate method is dictated from the size, location, histological subtype of LCs and patient’s performance status including respiratory function and/or other comorbidities (3,8). Without compromising the oncological outcome, the preservation of as much normal lung tissue as possible should also be pursued.
Besides radical resection of the primary tumor, systematic hilar and mediastinal lymph node dissection should be an integral part of the operation, since the likelihood of nodal metastases is present in either types of LCs albeit much higher in ACs. Lymphadenectomy may be only omitted if a rigorous mediastinal node sampling has been preoperatively performed in TCs.
Adjuvant therapy
Adjuvant treatment in the form of chemotherapy is common practice in limited-stage SCLC or in the early stages of NSCLC. By contrast, the role of adjuvant therapy in LCs is controversial. There are currently no prospective randomized trials investigating whether patients with LCs are benefited from post-operative treatment, and this lack of studies is mostly attributed to the low incidence of these tumors. Hence, current recommendations existing regarding adjuvant therapy, are either based on retrospective studies or arise from extrapolation from studies in more advanced stages of disease.
Especially for the resectable low-grade TCs, adjuvant chemotherapy seems to confer no survival benefit, even when lymph nodes are involved. According to a recent large study of Nussbaum et al., no survival advantage was demonstrated at 5 years when adjuvant chemotherapy was administered after lobectomy for TC (9). Despite the limitations of this study, these results are in line with current recommendations, suggesting only observation after surgery (10). On the contrary, the management of resectable intermediate-grade ACs requires a more multimodal approach, in the context of which cytotoxic treatment may be beneficial for some patients. The more aggressive biology of this tumor, more frequent presence of nodal metastases, higher relapse rates and the worse overall survival (OS) rates justify the rationale for adjuvant chemotherapy (11). Because of the very low incidence of ACs, the efficacy of this strategy has been assessed only in small case series and case reports, suggesting positive impact on survival (12).
Within this spectrum of inadequate evidence, the National Comprehensive Cancer Network (NCCN) suggests adjuvant chemotherapy could be considered, with or without radiotherapy, in resectable stage IIIA ACs (10), while the European Neuroendocrine Tumor Society (ENETS) expands this consideration in patients with ACs metastasized to lymph nodes (3). The not so recent North American Neuroendocrine Tumor Society (NANETS) guidelines do not recommend adjuvant treatment (13).
Based on improved response rates (RR) in the metastatic setting, the combination of a platinum derivative with etoposide comprises the recommended regimen in adjuvant chemotherapy. The immunohistochemical expression of the proliferation index ki-67 and the number of mitoses in ACs may guide the decision for adjuvant chemotherapy, since greater values of these parameters imply that the tumor is more chemosensitive. However, this hypothesis needs to be proven in clinical trials.
Notably, other forms of systemic treatment such as targeted therapies have recently been introduced in more advanced stages of LCs. Therefore, resectable LCs with high-risk features such as atypical histology, large size or lymph node metastases highlight the need for trials in which novel agents like everolimus will be tried in the adjuvant setting. Cooperation among specialized institutions is essential, so that well-designed clinical trials with large and homogeneous populations will be conducted. Until then, the implementation of adjuvant therapy should be decided on an individualized basis and after discussion in multidisciplinary tumor board meetings.
Metastatic disease
A small proportion (up to 3%) of LCs are first diagnosed when already have metastasized. The property of early dissemination characterizes mainly the atypical variant of LCs (3% for TCs and 21% for ACs), according to a large analysis of approximately 142 LCs (14). The most frequent site of distant metastatic disease is the liver and other sites include bone, adrenal glands, and brain. The management of progressive or de novo metastatic LCs aims at prolongation of survival and palliation of symptoms and is mostly based on systemic therapies. In the next paragraphs, we list the available systemic treatments in this rare but with adverse prognosis, subgroup of LCs and emphasize on more recent data regarding especially targeted therapy, while current recommendations are summarized in Table 1.
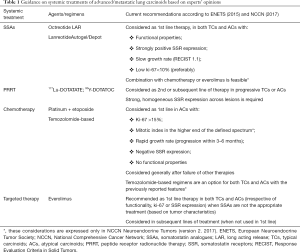
Full table
SSAs
SSAs comprise a classic treatment for malignant carcinoid syndrome and are frequently included in the therapeutic strategy of NETs. SSAs are synthetic analogues of somatostatin with longer half-lives than the endogenous hormone and act through activation of somatostatin receptors (SSRs). The most commonly used agents are octreotide and lanreotide. According to preclinical and clinical studies of the last 2 decades, SSAs exert dual inhibitory action, against both hormone secretion and proliferation of cells (15,16).
For many years, the inhibitory effect of SSAs on the excretion of neurosecretory peptides and the improved quality of life for patients with carcinoid syndrome had established the use of these agents against functional NETs, while their efficacy in non-functional tumors was questionable. This hypothesis was tested by two prospective phase 3 randomized trials—PROMID and CLARINET. In PROMID trial, the long acting release (LAR) formulation of octreotide 30 mg was found to significantly lengthen progression-free survival (PFS) compared with placebo in patients with both functionally active and inactive metastatic midgut NETs (17). Similarly, in CLARINET trial, lanreotide was associated with significantly prolonged time to progression (TTP) among patients with metastatic grade 1 or 2, non-functioning enteropancreatic tumors (18). It’s obvious that these two trials proved the antiproliferative activity of SSAs and, despite the limitation of being conducted in gastroenteropancreatic NETs (GEP-NETs), created the rationale for use in non-functioning NETs of other origins.
As far as LCs are concerned, functionality is not a typical feature. Moreover, the benefit of SSAs in the advanced stages has not been confirmed in prospective randomized trials and supporting evidence arises only from retrospective studies (19,20).
Slowly growing LCs with low proliferative index (Ki-67), high positivity for SSR scintigraphy (Octreoscan) or functioning properties may show which patients with LCs are most likely to benefit from treatment with SSAs. This knowledge that has been acquired from studies in GEP-NETs seems applicable to progressive, metastatic LCs according to a recent retrospective study (21).
To date, two ongoing clinical trials—SPINET and ATLANT–are testing lanreotide in unresectable LCs. SPINET (NCT02683941) is a phase 3, multi-center, randomized, double-blind study with intention to show the effect of lanreotide vs. placebo (22), whereas ATLANT is a phase 2, multicentre, single arm, open-label trial that will evaluate efficacy and safety of the combination of lanreotide with chemotherapy (temozolomide).
SSR type 2A (SSR-2A) is the most frequently expressed among the five members of this receptor family and comprises the main target for classic SSAs. Nevertheless, recent research has shown that SSR-1, SSR-3 and SSR-5 are additionally expressed in lung NETs, in quite different but clinically meaningful rates and suggests the possible therapeutic implication of these SSRs (23,24).
An ideal molecule targeting more SSRs is pasireotide—a multi-receptor ligand SSA with high binding affinity for many SSRs (types 1, 2, 3, 5)—that has currently been approved in acromegaly and Cushing’s disease. Pasireotide is already evaluated in patients with LCs who participate in a multicenter phase 2 trial with three interventional arms. (LUNA trial, NCT01563354) (25). In conclusion, SSAs administration is critical in patients with advanced LCs that present with paraneoplastic manifestations. In the remainder of LCs without hormonal symptoms, SSAs may be given, especially in cases of a highly positive Octreoscan. The ongoing prospective studies will determine the effectiveness and safety of these drugs in progressive, non-functional LCs. The combination of SSAs with other targeted agents seems a very promising approach.
Peptide receptor radionuclide therapy (PRRT)
PRRT is a therapeutic application of nuclear medicine with significant contribution to the management of advanced NETs. PRRT could be described as a kind of systemic radiotherapy, that uses radiolabelled SSAs. Due to their unique conjugation, these drugs target neoplastic cells that express SSRs, and emit radiation to them via a radioisotope. 177Lu-DOTATATE and 90Y-DOTATOC are the two representative compounds of PRRT and contain Lutetium-177 and Yttrium-90, respectively.
Until recently, the experience with PRRT in the management of LCs was very limited, as relevant data were derived from general studies on NETs, including a minor percentage of LCs. In the large series of Imhof et al., 1,109 patients with various NETs participated and a 28.6% overall response rate (ORR) was shown in the subgroup of 84 LCs treated with 90Y-DOTATOC (26). Similarly, administration of 177Lu-DOTATATE achieved 30% ORR and an additional 30% stable disease (SD) in the lung cohort (23 patients) of another large series of NETs (27).
Two recently published studies have investigated the efficacy of PRRT against LCs exclusively. The first one retrospectively evaluated ORR, OS and PFS in 114 patients with advanced LCs, applying three different PRRT protocols (90Y-DOTATOC vs. 177Lu-DOTATATE vs. 90Y-DOTATOC plus 177Lu-DOTATATE). According to the results, median OS (estimated in 94 patients) was 58.8 months, median PFS was 28 months and a 26.5% ORR was observed in the entire cohort. Despite the highest ORR (38.1%) with the combined administration, treatment with single 177Lu-DOTATATE achieved the highest 5-year OS (61.4%) (28). In the second study, prospective phase 2 trial enrolling 34 patients with progressive LCs-PRRT with177Lu-DOTATATE resulted in 15% ORR and 47% SD as well as median PFS and OS of 19 and 49 months, respectively (29).
Accordingly, PRRT may be considered in cases of progressive LCs with intense SSR expression across lesions. There is no comparative study of PRRT against SSAs regarding LCs, but the randomized controlled phase 3 NETTER-1 trial—comparing 177Lu-DOTATATE vs. high-dose octreotide LAR in metastatic progressive (after SSA first-line treatment) midgut NETs—revealed improved PFS and higher ORR for PRRT arm (30). Thus NETTER-1 may indirectly favor PRRT in advanced lines of LC treatment.
Chemotherapy
LCs are generally tumors with low proliferative capacity and cytotoxic chemotherapy as expected has limited activity. However, chemotherapy remains an option for palliative treatment in patients with metastatic LCs. While several chemotherapeutic drugs either alone or in combination have been tested in NETs, just a few studies have investigated exclusively the benefit of chemotherapy in LCs (12,19,31).
The spectrum of chemotherapy that has been reported in this setting consists of 5-fluorouracil (5-FU), capecitabine, doxorubicin, dacarbazine, streptozotocin, cyclophosphamide, platinum derivatives, etoposide and temozolomide, with all these drugs showing modest (<30% ORR) efficacy when administered in various combinations.
The etoposide-platinum regimens which compose the treatment of choice in poorly differentiated lung NETs (SCLC, LCNELC), have also shown anti-tumor activity in small case series of patients with LCs. In one such retrospective study, 17 patients with advanced LCs were treated with etoposide with either cisplatin or carboplatin and 4 of them (23.5%) achieved radiological response (32). Similar RR (23%) are reported in another retrospective study of Chong et al., that evaluated the same chemotherapy regimen exclusively in ACs (13 patients). One of the three responses was complete and was observed after carboplatin plus etoposide treatment. Moreover, the clinical benefit of this platinum-based chemotherapy in this study was very high, as additional to responses, nine cases (69%) presented SD (12).
Another appealing chemotherapeutic agent in the management of LCs is temozolomide, which is an orally administered drug with low toxicity profile. The first study suggesting the effectiveness of temozolomide monotherapy against LCs was a retrospective one and among other NETs, included 13 patients with LCs—10 TCs and 3 ACs—that received temozolomide in second or subsequent lines of therapy. Of them, eight patients (62%) derived clinical benefit—4 partial responses (PR) and 4 SD. Remarkably the 3 PR were noticed in TCs (33). Similar results are reported in a larger retrospective cohort with 31 metastatic LCs. Clinical benefit of temozolomide monotherapy was shown in 14—3 PR and 11 SD—of the 22 patients in whom response was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). Particularly, the 3 PR occurred in patients with ACs and high Ki-67 proliferative index (31).
In addition, a few retrospective studies and one prospective phase 2 trial have investigated the synergistic effect of capecitabine-temozolomomide (CAPTEM) combination against a variety of NETs, including LCs. Results from an interim analysis of this specific phase 2 trial, revealed that CAPTEM led to 1 complete response (CR), 4 PR, 7 SD and a greater than 22 months median PFS in the cohort of 12 patients with progressive LCs (34).
Despite the low level of existing evidence, results from retrospective studies influence large cooperative oncology groups to suggest chemotherapy in cases where every other systemic treatment has failed. Especially ENETS guidelines set additional recommendations regarding chemotherapy use, such as the cut-off value of proliferative index (Ki-67 >15%) for ACs, the rapid disease progression (according to RECIST criteria) or the absence of SSR expression (35). According to NCCN, temozolomide is an option in all metastatic LCs irrespective of grade, while the combination of a platinum agent with etoposide should be considered for the highly proliferating ACs (10).
Co-administration of chemotherapy with SSAs is also reported as therapeutic approach in NCCN guidelines. However, no trials so far have investigated the effects of combining chemotherapy with other systemic treatments in LCs. As mentioned before, ATLANT is the only phase 2 clinical trial (NCT02698410) in the accrual period, that aims to define efficacy and safety of lanreotide in combination with temozolomide in patients with unresectable advanced lung and thymic NETs.
Molecular-targeted therapy
A significant progress in the management of progressive advanced LCs has been made with the introduction of everolimus—a mammalian target of rapamycin (mTOR) inhibitor—in clinical practice. The first signs of its potential efficacy had already been presented in a randomized phase 3 trial (RADIANT-2) that assessed the efficacy and safety of everolimus plus octreotide LAR vs. placebo plus octreotide LAR, in advanced functional well to moderately differentiated NETs of diverse primary sites. The exploratory subgroup analysis of this study in the 44 patients with LCs favored the addition of everolimus to octreotide LAR (improved PFS), even though not reaching statistical significance because of the limited number of cases (36). Similarly, another phase 2 study (RAMSETE) demonstrated high disease control rate (DCR) and favorable PFS in patients with non-functional, non-pancreatic NETs (including LCs) treated with everolimus monotherapy (37).
Thereafter, systemic treatment with everolimus received FDA approval in 2016 based upon RADIANT-4—a randomized, double-blind, placebo-controlled, phase 3 trial. RADIANT-4 evaluated the efficacy and safety of everolimus compared with placebo in 302 patients with gastrointestinal or pulmonary non-functional NETs. Among the study population, 90 patients with LCs were included. RADIANT-4 met its primary outcome measure, indicating significant increase in median PFS {11 and 3.9 months in everolimus and placebo arms respectively [hazard ratio (HR), 0.48; 95% CI, 0.35–0.67], P<0.001} (38). The subsequent subgroup analysis in LCs continued showing improved PFS [9.2 vs. 3.6 months (HR, 0.50; 95% CI, 0.28–0.88)], establishing the role of targeted therapy in LCs (39).
Combination of everolimus with SSAs is under investigation in LUNA trial. The aim of this 3-arm study is to compare the combination of everolimus plus pasireotide LAR with either agent alone, in advanced LCs as well as thymic carcinoids. Preliminary results have already been published, showing promising PFS rates at month 9–39% (for pasireotide LAR), 33.3% (for everolimus) and 58.5% (for the combined administration) (40).
Beyond mTOR inhibition, treatment strategies with angiogenesis blockade have been tested in mixed NET populations, though with mostly unsuccessful outcomes. Firstly, bevacizumab—a monoclonal antibody against VEGF—was compared to pegylated interferon (PEG-IFN) to define which one creates the more effective combination with octreotide LAR in the treatment of advanced NETs. Despite the initial favorable results for bevacizumab in a randomized phase 2 study (41), this anti-VEGF targeted agent failed to show improved PFS in the following randomized phase 3 trial (42). The co-administration of bevacizumab with chemotherapy and/or other targeted agents has been extensively studied in several phase 2 trials, including patients with pancreatic NETs (pNETs) or gastrointestinal carcinoids, while no data exist in LCs. In a randomized phase 2 study of pNETs, the combination of bevacizumab plus everolimus resulted in increased RR and greater (not statistically significant) PFS but was accompanied with higher frequency and severity of adverse events compared to everolimus monotherapy (43). Maybe this therapeutic approach deserves investigation in advanced LCs.
Sunitinib—an oral multi-targeted receptor tyrosine kinase (RTK) inhibitor with antiangiogenic activity—was established as standard treatment in patients with pNETs after its great improvements in survival and RR in the randomized phase 3 trial of Raymond et al. (44). Unlike pNETs, there is lack of evidence regarding the role of sunitinib in the management of LCs. The only relevant data came from the single arm phase 2 study of Kulke et al., even though no information about the exact number of patients with LCs was provided, since they were classified together with stomach carcinoids as foregut carcinoids (45). In particular, 14 foregut carcinoids were included in the cohort of 40 patients with gastrointestinal carcinoids. While only 1 response in this cohort was recorded, there was a remarkably high overall DCR (82.9%) among carcinoid patients. These results underlined the tumoristatic rather than the cytotoxic effect of sunitinib and suggested further investigation in randomized trials so as to define the clear benefit of sunitinib in carcinoid tumors, including LCs. For the time being, efficacy of sunitinib remains undetermined in LCs and further prospective studies are warranted.
On the other hand, pazopanib—an oral multi-targeted agent against VEGFR-1, -2 and -3, PDGFR α and β and c-Kit—was tried in a phase 2 (PAZONET) study as sequential therapy in 42 patients with progressive NETs (including 5 LCs). The majority of patients had previously received mTOR and other protein kinase inhibitors. This trial showed inferior activity of pazopanib in the subgroup of lung and thymic carcinoids—median PFS: 3.4 months (95% CI, 0.0–7.0 months)—compared to gastrointestinal carcinoids and pNETs (46). When combined with depot octreotide in another phase 2 study, pazopanib failed to yield responses in the cohort of carcinoid tumors (1 LC), in contrast to the cohort of pNETs, where few PR were detected (47).
Apparently, the success of mTOR inhibition has introduced the era of molecularly targeted therapy in LCs. Numerous targeted agents, especially tyrosine kinase inhibitors (regorafenib, nintedanib, sulfatinib) or even drugs used in hematological malignancies (carfilzomib, ibrutinib) are currently evaluated in clinical trials, involving mixed populations with unresectable or metastatic LCs and GEP-NETs.
LCNELC
LCNELC is a poorly differentiated tumor containing cells of large size with neuroendocrine morphology and expression. The previous categorization of LCNELC as a specific variant of pulmonary large cell carcinoma, was revised by the 2015 WHO classification and LCNELC currently belongs to the group of lung NETs (2). Except for its low incidence, the diagnosis of LCNELC cannot be safely established from the histological examination of core biopsy samples and requires surgical specimens. Thus, we assume that many LCNELC, not amenable to surgery are misdiagnosed as undifferentiated NSCLC or other large cell carcinomas. Compared to SCLC, LCNELC is characterized by mainly peripheral location and more frequently presents as localized, potentially resectable tumor. However, LCNELC is generally an aggressive cancer with poor prognosis and not so significant improvements in its management so far. In the next paragraphs, we point out current treatment strategies and future perspectives for this difficult clinical entity.
Resectable disease
In cases of localized or even locally advanced LCNELC (stage I–IIIA), complete surgical resection with hilar and mediastinal lymph node dissection should be performed, if there are no contraindications for surgery. Given the high rates of recurrence after surgery and poor prognosis even in early stage disease—33% (5-year OS rate) in stage I according to retrospective data (48)—a multimodal approach has been proposed as curative strategy. The effect of adjuvant chemotherapy has been investigated in several retrospective and a few prospective studies with limited number of patients.
Encouraging results regarding the impact of platinum—etoposide adjuvant chemotherapy was first provided by a retrospective analysis of Rossi et al. (48). Two additional retrospective studies followed, confirming prolonged survival with various regimens of adjuvant chemotherapy (49), while a European multicenter study (including 400 patients) revealed just a trend towards benefit (50).
The first prospective analysis was conducted by Iyoda et al. and involved 15 patients with LCNELC who had previously undergone complete surgical resection. These patients received up to two courses of adjuvant chemotherapy with the standard combination regimen of SCLC (cisplatin plus etoposide). The comparison of survival between the interventional group and historical control group was clearly in favor of the platinum-based chemotherapy (5-year OS rate: 88.9% vs. 47.4% respectively) (51).
Moreover, promising results in the adjuvant setting are reported with cisplatin plus irinotecan, as a multicenter phase 2 pilot study showed that four cycles of post-operative administration in 23 patients with LCNELC could lead to an 86% OS rate and 74% relapse-free survival rate at 3 years of follow-up (52). Efficacy and safety of this regimen have extensively been assessed in extended SCLC and non-inferiority in terms of survival has been demonstrated when compared with platinum plus etoposide. These data create the background for the ongoing Japanese multicenter randomized phase 3 study (JCOG1205/1206) that compares the two platinum-based regimens as adjuvant treatment in high-grade neuroendocrine carcinomas of the lung. Among others, the results of this trial are expected to elucidate which chemotherapy is more active in the subgroup of patients with LCNELC (53).
In addition to chemotherapy, there is preliminary evidence supporting the beneficial impact of SSAs as post-operative treatment in cases of positive Octreoscan before surgery (54). SSAs have a much more tolerable side-effect profile compared to chemotherapy. However, their precise role in the adjuvant setting should be investigated in randomized clinical trials (SSAs versus chemotherapy), involving patients with SSR over-expressing tumors.
Metastatic disease
Metastatic LCNELC is a lethal disease, the management of which remains controversial. Platinum-based chemotherapy is the most widely adopted systemic intervention, but the optimal platinum combination is still a matter of discussion. Over the last decade a trend has been observed towards treating (first-line) metastatic LCNELC in the same way as SCLC, rather than as NSCLC. This is supported by mainly retrospective studies with favorable results for SCLC treatments, while the rationale for this kind of treatment was based on the common neuroendocrine features and aggressive biology of both histologic subtypes.
The initial, somehow practice-changing study was conducted by Rossi et al. and reported statistically significant superiority of platinum/etoposide treatment in terms of OS vs. NSCLC regimens in both adjuvant and metastatic setting of totally 83 retrospectively reviewed pure LCNELC (48). Nevertheless, subsequent studies came up with conflicting results (55,56). Notably, negative comparative outcomes from the administration of SCLC chemotherapy have just been published in a large retrospective cohort with pathology-reviewed advanced LCNELC. In this study, with the exception of platinum/pemetrexed, a statistical significant improvement is shown in median (95% CI) OS with NSCLC chemotherapy [8.5 months (7.0–9.9 months)] compared to SCLC treatment [6.7 months (5.0–8.5 months); P=0.012] (57).
While the efficacy and safety of the combined administration of cisplatin with etoposide and of cisplatin with irinotecan have been assessed in advanced LCNELC in prospective phase 2 studies with ORR of 34% and 47% respectively, no prospective randomized trials have been conducted so far comparing these regimens with NSCLC chemotherapy (58,59). Only this kind of trials can provide strong level of evidence in order to answer which chemotherapy is more effective.
Additionally, administration of amrubicin—an anthracycline derivative marketed in Japan—as a single agent in the second line, has been described in two retrospective studies with small sample size and revealed limited efficacy (ORR: 11.1–27.7%, DCR: 61.1% in both studies) (60,61). Furthermore, nedaplatin—a newer platinum derivative—co-administered with irinotecan showed promising effectiveness and safety in a retrospective analysis of 18 chemonaive patients with LCNELC (localized and advanced disease), but no prospective validation of this combination has been performed so far (62).
Despite its cytotoxic effects that many times are translated into tumor response, chemotherapy offers modest benefit and remains far from changing the natural history of advanced LCNELC. Thus, the discovery of more potent novel compounds is needed. Unfortunately, LCNELC belongs to the group of tumors that do not typically harbor targetable oncogenic driver mutations. Preliminary evidence from molecular screening and immunohistochemical analyses of LCNELC suggests possible role of precision treatments against VEGF, c-KIT and HER-2 signaling pathways (63).
Finally, based on the findings of mTOR inhibition in neuroendocrine neoplasms, a prospective multicenter phase 2 trial assessed the efficacy and safety of the addition of everolimus to carboplatin and paclitaxel in 49 patients with metastatic LCNELC. Despite the premature discontinuation of the study due to low enrollment rate, promising results emerged such as the high PFS rate of 76% at 3 months and the median OS of 9.9 months, with acceptable frequency and severity of adverse events (64). The detection of predictive biomarkers such as specific molecular alterations of the PI3K/AKT/mTOR pathway, is essential so that we distinguish which patients are going to benefit from this combined therapy.
Conclusions
The treatment landscape in the management of advanced LCs broadens after the recent success of everolimus in improving PFS, while SSAs, chemotherapy and PRRT remain potentially effective options when used based on specific tumor features. However, the optimal sequence of these therapies and the feasibility of combinations have not yet been defined. Additionally, numerous novel targeted agents are currently evaluated in clinical trials on well-differentiated NETs and useful results may arise in the near future regarding LCs. By contrast, no clinical research has been conducted so far on the role of immunotherapy. In the future, prospective randomized trials conducted exclusively on patients with LCs are warranted in order to minimize bias and obtain high level of evidence. Given the low incidence of these tumors, cooperation among specialized institutions is essential for enrolling adequate numbers of patients.
As far as LCNELC is concerned, this is an aggressive lung NET for which chemotherapy remains the gold standard of therapy. Even though regimens used in SCLC are more popular in its treatment, randomized trials are needed to elucidate if chemotherapy used in NSCLC is more or less beneficial. Combinations of chemotherapy with targeted therapy or implementation of immunotherapy should comprise the issues of forthcoming clinical research.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010;134:1628-38. [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol 2015;26:1604-20. [Crossref] [PubMed]
- Fasano M, Della Corte CM, Papaccio F, et al. Pulmonary Large-Cell Neuroendocrine Carcinoma: From Epidemiology to Therapy. J Thorac Oncol 2015;10:1133-41. [Crossref] [PubMed]
- Marquez-Medina D, Popat S. Systemic therapy for pulmonary carcinoids. Lung Cancer 2015;90:139-47. [Crossref] [PubMed]
- Detterbeck FC. Clinical presentation and evaluation of neuroendocrine tumors of the lung. Thorac Surg Clin 2014;24:267-76. [Crossref] [PubMed]
- Travis WD, Giroux DJ, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2008;3:1213-23. [Crossref] [PubMed]
- Oberg K, Hellman P, Kwekkeboom D, et al. Neuroendocrine bronchial and thymic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v220-2. [Crossref] [PubMed]
- Nussbaum DP, Speicher PJ, Gulack BC, et al. Defining the role of adjuvant chemotherapy after lobectomy for typical bronchopulmonary carcinoid tumors. Ann Thorac Surg 2015;99:428-34. [Crossref] [PubMed]
- National Comprehensive Cancer Network NCCN. Clinical Practice Guidelines in Oncology: Neuroendocrine Tumors. 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- Garcia-Yuste M, Matilla JM, Cueto A, et al. Typical and atypical carcinoid tumours: analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung. Eur J Cardiothorac Surg 2007;31:192-7. [Crossref] [PubMed]
- Chong CR, Wirth LJ, Nishino M, et al. Chemotherapy for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer 2014;86:241-6. [Crossref] [PubMed]
- Phan AT, Oberg K, Choi J, et al. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas 2010;39:784-98. [Crossref] [PubMed]
- Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001;119:1647-51. [Crossref] [PubMed]
- Aparicio T, Ducreux M, Baudin E, et al. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer 2001;37:1014-9. [Crossref] [PubMed]
- Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol 2006;17:1733-42. [Crossref] [PubMed]
- Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. [Crossref] [PubMed]
- Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224-33. [Crossref] [PubMed]
- Granberg D, Eriksson B, Wilander E, et al. Experience in treatment of metastatic pulmonary carcinoid tumors. Ann Oncol 2001;12:1383-91. [Crossref] [PubMed]
- Srirajaskanthan R, Toumpanakis C, Karpathakis A, et al. Surgical management and palliative treatment in bronchial neuroendocrine tumours: a clinical study of 45 patients. Lung Cancer 2009;65:68-73. [Crossref] [PubMed]
- Sullivan I, Le Teuff G, Guigay J, et al. Antitumour activity of somatostatin analogues in sporadic, progressive, metastatic pulmonary carcinoids. Eur J Cancer 2017;75:259-67. [Crossref] [PubMed]
- Reidy-Lagunes D, Kulke M, Wolin E, et al. PUB119 Lanreotide in Patients with Lung Neuroendocrine Tumors: The Randomized Double-Blind Placebo-Controlled International Phase 3 SPINET Study. J Thorac Oncol 2017;12:S1516-7. [Crossref]
- Kanakis G, Grimelius L, Spathis A, et al. Expression of Somatostatin Receptors 1-5 and Dopamine Receptor 2 in Lung Carcinoids: Implications for a Therapeutic Role. Neuroendocrinology 2015;101:211-22. [Crossref] [PubMed]
- Righi L, Volante M, Tavaglione V, et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 'clinically aggressive' cases. Ann Oncol 2010;21:548-55. [Crossref] [PubMed]
- 3-arm Trial to Evaluate Pasireotide LAR/Everolimus Alone/in Combination in Patients With Lung/Thymus NET - LUNA Trial. Available online: https://ClinicalTrials.gov/show/NCT01563354
- Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29:2416-23. [Crossref] [PubMed]
- Brabander T, van der Zwan WA, Teunissen JJM, et al. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res 2017;23:4617-24. [Crossref] [PubMed]
- Mariniello A, Bodei L, Tinelli C, et al. Long-term results of PRRT in advanced bronchopulmonary carcinoid. Eur J Nucl Med Mol Imaging 2016;43:441-52. [Crossref] [PubMed]
- Ianniello A, Sansovini M, Severi S, et al. Peptide receptor radionuclide therapy with (177)Lu-DOTATATE in advanced bronchial carcinoids: prognostic role of thyroid transcription factor 1 and (18)F-FDG PET. Eur J Nucl Med Mol Imaging 2016;43:1040-6. [Crossref] [PubMed]
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125-35. [Crossref] [PubMed]
- Crona J, Fanola I, Lindholm DP, et al. Effect of temozolomide in patients with metastatic bronchial carcinoids. Neuroendocrinology 2013;98:151-5. [Crossref] [PubMed]
- Forde PM, Hooker CM, Boikos SA, et al. Systemic therapy, clinical outcomes, and overall survival in locally advanced or metastatic pulmonary carcinoid: a brief report. J Thorac Oncol 2014;9:414-8. [Crossref] [PubMed]
- Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res 2007;13:2986-91. [Crossref] [PubMed]
- Fine RL, Gulati AP, Tsushima D, et al. Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated metastatic neuroendocrine tumors. J Thorac Oncol 2014;32:179.
- Pavel M, O'Toole D, Costa F, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016;103:172-85. [Crossref] [PubMed]
- Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011;378:2005-12. [Crossref] [PubMed]
- Pavel ME, Wiedenmann B, Capdevila J, et al. RAMSETE: A single-arm, multicenter, single-stage phase II trial of RAD001 (everolimus) in advanced and metastatic silent neuro-endocrine tumours in Europe. J Thorac Oncol 2012;30:4122.
- Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016;387:968-77. [Crossref] [PubMed]
- Yao J, Fazio N, Buzzoni R, et al. ORAL02.02: Efficacy and Safety of Everolimus in Advanced, Progressive, Nonfunctional Neuroendocrine Tumors (NET) of the Lung: RADIANT-4 Subgroup Analysis: Topic: Medical Oncology. J Thorac Oncol 2016;11:S253. [Crossref] [PubMed]
- Ferolla P, Brizzi MP, Meyer T, et al. Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol 2017;18:1652-64. [Crossref] [PubMed]
- Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol 2008;26:1316-23. [Crossref] [PubMed]
- Yao JC, Guthrie KA, Moran C, et al. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients With Advanced Carcinoid Tumors: SWOG S0518. J Clin Oncol 2017;35:1695-703. [Crossref] [PubMed]
- Kulke MH, Niedzwiecki D, Foster NR, et al. Randomized phase II study of everolimus (E) versus everolimus plus bevacizumab (E+B) in patients (Pts) with locally advanced or metastatic pancreatic neuroendocrine tumors (pNET), CALGB 80701 (Alliance). J Thorac Oncol 2015;33:4005.
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [Crossref] [PubMed]
- Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 2008;26:3403-10. [Crossref] [PubMed]
- Grande E, Capdevila J, Castellano D, et al. Pazopanib in pretreated advanced neuroendocrine tumors: a phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann Oncol 2015;26:1987-93. [Crossref] [PubMed]
- Phan AT, Halperin DM, Chan JA, et al. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: a multicentre, single-group, phase 2 study. Lancet Oncol 2015;16:695-703. [Crossref] [PubMed]
- Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005;23:8774-85. [Crossref] [PubMed]
- Saji H, Tsuboi M, Matsubayashi J, et al. Clinical response of large cell neuroendocrine carcinoma of the lung to perioperative adjuvant chemotherapy. Anticancer Drugs 2010;21:89-93. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Evangelista A, et al. Adjuvant chemotherapy for large-cell neuroendocrine lung carcinoma: results from the European Society for Thoracic Surgeons Lung Neuroendocrine Tumours Retrospective Database. Eur J Cardiothorac Surg 2017;52:339-45. [PubMed]
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg 2006;82:1802-7. [Crossref] [PubMed]
- Kenmotsu H, Niho S, Ito T, et al. A pilot study of adjuvant chemotherapy with irinotecan and cisplatin for completely resected high-grade pulmonary neuroendocrine carcinoma (large cell neuroendocrine carcinoma and small cell lung cancer). Lung Cancer 2014;84:254-8. [Crossref] [PubMed]
- Eba J, Kenmotsu H, Tsuboi M, et al. A Phase III trial comparing irinotecan and cisplatin with etoposide and cisplatin in adjuvant chemotherapy for completely resected pulmonary high-grade neuroendocrine carcinoma (JCOG1205/1206). Jpn J Clin Oncol 2014;44:379-82. [Crossref] [PubMed]
- Filosso PL, Ruffini E, Oliaro A, et al. Large-cell neuroendocrine carcinoma of the lung: a clinicopathologic study of eighteen cases and the efficacy of adjuvant treatment with octreotide. J Thorac Cardiovasc Surg 2005;129:819-24. [Crossref] [PubMed]
- Sun JM, Ahn MJ, Ahn JS, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer 2012;77:365-70. [Crossref] [PubMed]
- Naidoo J, Santos-Zabala ML, Iyriboz T, et al. Large Cell Neuroendocrine Carcinoma of the Lung: Clinico-Pathologic Features, Treatment, and Outcomes. Clin Lung Cancer 2016;17:e121-9. [Crossref] [PubMed]
- Derks JL, van Suylen RJ, Thunnissen E, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinomas: does the regimen matter? Eur Respir J 2017;49. [Crossref] [PubMed]
- Niho S, Kenmotsu H, Sekine I, et al. Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: a multicenter phase II study. J Thorac Oncol 2013;8:980-4. [Crossref] [PubMed]
- Le Treut J, Sault MC, Lena H, et al. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: the GFPC 0302 study. Ann Oncol 2013;24:1548-52. [Crossref] [PubMed]
- Kasahara N, Wakuda K, Omori S, et al. Amrubicin monotherapy may be an effective second-line treatment for patients with large-cell neuroendocrine carcinoma or high-grade non-small-cell neuroendocrine carcinoma. Mol Clin Oncol 2017;6:718-22. [Crossref] [PubMed]
- Yoshida H, Sekine I, Tsuta K, et al. Amrubicin monotherapy for patients with previously treated advanced large-cell neuroendocrine carcinoma of the lung. Jpn J Clin Oncol 2011;41:897-901. [Crossref] [PubMed]
- Kenmotsu Y, Oshita F, Sugiura M, et al. Nedaplatin and irinotecan in patients with large-cell neuroendocrine carcinoma of the lung. Anticancer Res 2012;32:1453-6. [PubMed]
- Iyoda A, Travis WD, Sarkaria IS, et al. Expression profiling and identification of potential molecular targets for therapy in pulmonary large-cell neuroendocrine carcinoma. Exp Ther Med 2011;2:1041-5. [Crossref] [PubMed]
- Christopoulos P, Engel-Riedel W, Grohe C, et al. Everolimus with paclitaxel and carboplatin as first-line treatment for metastatic large-cell neuroendocrine lung carcinoma: a multicenter phase II trial. Ann Oncol 2017;28:1898-902. [Crossref] [PubMed]


