Quality in upper gastrointestinal endoscopic submucosal dissection
Introduction
The diagnosis of early neoplastic lesions of the gastrointestinal (GI) tract has increased in the last two decades thanks to the widespread use of GI endoscopy and to the progress and innovation of the endoscopic technology field. For many years, surgery was the treatment of choice for superficial, early stage GI tumors but it was associated to high morbidity rate and reduced quality of life (1). In order to avoid invasive surgery and, therefore, to allow the preservation of the native organ, advanced endoscopic techniques have been developed to treat superficial GI lesions. Most early stage GI cancers can be treated by endoscopic mucosal resection (EMR), which involves the elevation of the lesion through the injection of EMR solution (usually a mixture of saline solution, indigo carmine and adrenaline) and then the resection of the lesion by using a polypectomy snare (2). GI lesions smaller than 20 mm in size can be safely removed en bloc with EMR, but bigger lesions (≥20 mm) usually require piecemeal EMR (EPMR), which is associated to a lower curative rate (3) and to a higher risk of recurrence (4,5). Moreover, even lesions smaller than 20 mm can be sometimes difficult to treat with EMR, because of their tricky location (such as behind a fold, on a corner or near a diverticulum) and/or for the presence of non-lifting sign (6). To overcome such limitations, in the mid-1990s, Japanese endoscopists developed endoscopic submucosal dissection (ESD), a technique that allows en bloc resection of early stage GI lesions, regardless of their shape and size. Since ESD was first performed, many scientific papers comparing ESD and EMR have been published in literature, with the aim of identifying statistically significant differences between the two procedures in terms of risks and benefits. In particular, a recent meta-analysis put together the results of several retrospective observational studies providing a total of 2,299 lesions (7). Although such studies were biased by differences in polyps’ size and location, results showed that en bloc resection rate and radical (R0) resection rate were higher and the recurrence rate was lower in the ESD group (91.7%, 80.3% and 0.9%, respectively) compared to the EMR group (46.7%, 42.3% and 12.2%, respectively). However, ESD is a technically difficult procedure, which requires a specialized training and a sufficient clinical expertise. Moreover, ESD requires a longer procedure time and is associated to a higher risk of perforation compared to EMR (5.7% for ESD versus 1.4% for EMR polypectomy) (7-9). ESD is now widely used in Asia for the treatment of early gastric cancer (EGC) (10-12) and colorectal malignant tumors (13), where it has been shown to be an effective and reasonably safe procedure. In Western countries ESD is not widely accepted yet, both because EGC is less common than in Asia, and therefore Western endoscopists are not provided with sufficient opportunities to start their training in easier locations (such as gastric antrum), and because an adequate training is still lacking. Nevertheless, in the last years, ESD has gained more attention among the Western endoscopists and it has been used by the trained ones for the treatment of both upper and lower GI lesions; this is also because of the technological improvement in the endoscopy field.
The aim of this paper is to provide endoscopists with a review about indications, limitations and technical aspects of upper GI ESD.
Eligibility criteria for ESD
The main difference between surgery and endoscopic treatment of superficial neoplastic lesions is the possibility to perform lymph node resection. Therefore, it is clear that endoscopic therapy can only be considered in case of lesions associated to a negligible risk of lymph node metastasis, or when the risk of metastasis is lower compared to the risk of mortality possibly related to surgery. The risk of lymph node metastasis is mostly associated to lesion’s depth of invasion, which, therefore, needs to be carefully evaluated before the procedure. Endoscopic evaluation of tumor’s depth varies upon the location along the GI tract and is based on particular imaging techniques, such as chromoendoscopy and virtual chromoendoscopy with magnification, which help in estimating not only the depth of invasion, but also lesion’s microsurface and microvascular pattern (14). However, the real depth of invasion can only be assessed through the histological analysis of the resected specimen, which is of main importance to identify high-risk features requiring surgery.
Esophagus
Squamous cell carcinoma (SCC)
Esophageal ESD should be performed only in highly specialized centers, as it is technically more difficult compared to ESD in the rest of the GI tract. Indeed, esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat (15). SCC is usually associated to a high risk of lymph node metastasis even with early lesions, because the lymphatics penetrate the muscularis mucosae, therefore an en bloc R0 resection is of main importance to obtain a better and longer disease-free survival. The size of the lesion is the main criterion to choose between EMR and ESD. EMR may be preferred for the removal of lesions <15 mm, as it usually has a safer profile compare to ESD (14). ESD can be considered the treatment of choice for lesions >15 mm, with poor lifting sign and for lesions possibly associated to submucosal invasion (14). However, a meta-analysis (16) showed that even for lesions <10 mm ESD should be preferred over EMR as the recurrence rate is lower with ESD. Based on such results, the European Society of Gastrointestinal Endoscopy (ESGE) suggests ESD to be the treatment of choice for any size SCC with negligible risk of lymph node metastasis and cap-assisted EMR (EMRc) to be used as an acceptable option for SCC <10 mm (17).
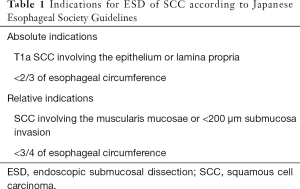
With respect to the Japanese Esophageal Society Guidelines for ESD of SCC, indications have been divided into two groups (18), as it is shown in Table 1:
- Absolute indications: intramucosal SCC involving the epithelium and lamina propria and occupying <2/3 of the esophageal circumference;
- Relative indications: SCC involving the muscularis mucosae or <200 µm invasion of the submucosa and occupying <3/4 of the esophageal circumference.
Full table
Circumferential involvement has to be carefully considered when choosing eligible candidates, as a defect involving more than 3/4 of the circumference may lead to esophageal stricture (19). However, some studies in literature (20,21) showed the effectiveness of oral and intralesional steroids administration to prevent stricture formation after ESD; therefore their use may overcome the size limitation.
Barrett’s esophagus (BE)-associated adenocarcinoma
So far, a very few data have been published in literature about ESD for the treatment of BE-associated adenocarcinoma (22-24), as in Asia BE and esophageal adenocarcinoma (EAC) are quite uncommon. On the contrary, in Western countries BE is a quite common diagnosis and EAC is the predominant esophageal malignancy. In the West, EMR for dysplastic BE associated to visible lesions has been shown to be safe and effective (25) and is therefore considered the treatment of choice (17). ESD should be considered only for lesions >15 mm, with poor lifting sign and when submucosal invasion is suspected (17).
Pre-procedural evaluation
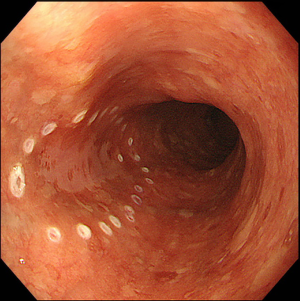
Lesions should be evaluated by an expert endoscopist with advanced imaging techniques in order to confirm the presence of invasive features and to accurately delineate the margins, as the size of the tumor and evidence of submucosal invasion will determine the choice between EMR and ESD. Not all the esophageal lesions are visible, as many of them present as flat, subtle neoplasia difficult to detect; however, high-resolution endoscopes are able to identify such lesions when used by an expert eye (26). Every visible lesion should be described according to Paris classification, as it is indicative of lesion’s malignant potential (27). In order to detect the lesion and carefully delineate the margins, chromoendoscopy has been shown to be very helpful. In particular, Lugol has been used to detect SCC (Figure 1) and acetic acid for BE, but it is important to underline that the outcome depends on the expertise and experience of the endoscopist. With the improvement of the endoscopic technology field, virtual chromoendoscopy with magnifying endoscopy has become available. It allows chromoendoscopy without the use of dyes and it is based either on light filters [Narrow Band Imaging (NBI), Olympus, Tokyo, Japan] or on digital processing after image acquisition [i-Scan, Pentax, Tokyo, Japan; Intelligent Chromoendoscopy (FICE), Fujinon, Saitama, Japan]. NBI is the most diffuse and used technique in the endoscopy field and also the most studied for the esophagus. With respect to SCC, it has been shown that NBI has the same sensitivity and superior specificity compared to Lugol (28), while for BE one study in literature showed that NBI has 96% sensitivity and 94% specificity for the diagnosis of high grade intraepithelial neoplasia (HGIN) (29). The evaluation of capillary loops with NBI is particularly important to estimate the invasive depth of esophageal lesions (30).
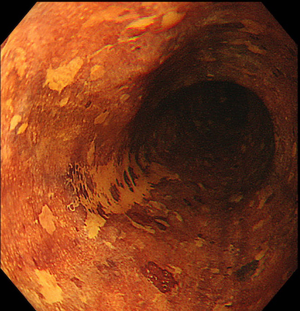
Once an accurate tumor assessment has been done, it is of main importance to confirm the presence of dysplasia through biopsies prior to endoscopic removal. There are no guidelines about the number of biopsies needed, but the general trend is toward as less biopsies as possible in order to minimize the risk of submucosal fibrosis, which may lead to a tricky dissection.
With respect to imaging examination, computed tomography (CT) scan and endoscopic ultrasonography (EUS) are usually not recommended before esophageal ESD. EUS should be considered only when submucosal invasion and/or lymph node involvement are suspected (17).
Outcomes for esophageal lesions
ESD for esophageal lesions has been shown to be safe, effective and able to reach an en bloc resection in a percentage of patients that varies between 85% and 100% (31,32). Moreover, compared to EMR, ESD has been shown to be superior in terms of complete resection, curative rate and recurrence free survival (1,33). Complication rate (including, bleeding, perforation and stricture formation) is higher with ESD than EMR, but patients usually recover completely (1,31).
Stomach
Gastric cancer is one of the most common neoplasia in Japan, where widespread screening programs led to early diagnosis and early treatment of such disease (34). Indeed, >50% of gastric cancer is diagnosed as EGC in Japan (35,36). EMR was the first endoscopic treatment to be developed as an alternative to surgery for EGC. Some studies showed that EMR was curative for about 85% of EGC cases (37,38) but was associated with a high rate of local recurrence (37). Later on, ESD was developed as an alternative to EMR and was shown to be associated to a higher rate of en bloc resection of bigger lesions and to a lower recurrence rate (39,40). Nevertheless, these better outcomes were associated to longer procedure time and higher risk of perforation as shown by two meta-analysis (41,42). However, no death was attributed to perforation in the aforesaid studies and authors concluded that ESD was better than EMR for the treatment of superficial gastric lesions, even though ESD has a slightly higher risk of perforation.
ESD is now considered by the Japanese guidelines as the treatment of choice for EGC with negligible risk of lymph node metastasis (11,43). With this respect, gastrectomy specimens have been analysed in order to find the relevant features related to the risk of lymph node metastasis (44,45), which are as follow: undifferentiated type, size >20 mm, submucosal invasion, lymphatic involvement, ulceration. Therefore, ESD is considered as an absolute indication in T1a differentiated-type lesions smaller than 20 mm, with no ulcerative findings (11). Expanded criteria for gastric ESD were then introduced in order to include those patients who were shown to have a low risk of lymph node metastasis, which outweighed the risk of surgery (46,47) (Table 2).

Full table
In Western countries, data on and experience in ESD for the treatment of gastric neoplastic lesions are still scanty, as the prevalence of gastric cancer is remarkably lower than in Asia. However, Western endoscopists performing ESD have now adopted the Japanese indications as shown in Table 2. ESGE recommends ESD as the treatment of choice for most superficial gastric lesions, with EMR being preferable only in cases of lesions <15 mm with a very low probability of advanced histology (0–IIa according to Paris classification) (17).
Pre-procedural evaluation
Prior to decide whether ESD is feasible or not, it is important to carefully assess the gastric lesion. Magnifying endoscopy with indigo carmine or virtual chromoendoscopy helps in diagnosing, staging and delineating tumor margins. Margins delineation can be tricky in undifferentiated EGC (48) because of the presence of proliferative zone extension (49); in such cases, underwater technique can be used together with magnification and NBI in order to improve the visualization of the demarcation line. Advanced imaging techniques are also useful to predict the histology type until a certain extent (50,51). However, histology type must be always determined by sample biopsies prior to endoscopic procedure. In particular, only one biopsy should be taken in order to minimize the risk of submucosal fibrosis; biopsies from the surrounding area are allowed only in case of undifferentiated EGC with indefinite margins (52).
EUS, CT scan or other imaging examination are not routinely recommended prior to gastric ESD. Indeed, endoscopic findings alone are usually accurate enough to predict the depth of invasion and tumor stage. In particular, findings associated with mucosal disease only, such as protrusion or depression of a smooth surface, modest elevation of the margins and smooth tapering of converting folds, are indicative of ESD feasibility. Therefore, ESGE suggests that an accurate endoscopic evaluation is sufficient to assess whether ESD is feasible or not, with EUS to be used only in selected cases (17). CT scan is usually not necessary, as the risk of lymph node metastasis for EGC considered suitable for ESD is extremely low (46,53).
Outcomes for gastric lesions
As already stated before, ESD is now considered the treatment of choice for EGC. Indeed, compared to EMR, ESD showed a higher en bloc and R0 resection rate and a lower risk of local recurrence (10,54). Data comparing ESD to surgery are limited. One retrospective study (55) showed that patients with EGC who underwent surgery had longer operative time and hospital stay, higher complication rate but similar oncological outcomes compared to ESD.
The largest series in literature on patients with EGC who underwent ESD showed a 5-year survival rate of 92.6%, a 5-year disease-specific survival rate of 99.9% and a 5-year relative survival rate of 105% (56). Local recurrence after curative gastric ESD is extremely unlikely and only one case has been reported in literature (57).
Duodenum
There are only a few data in literature about ESD for the treatment of duodenal lesions (58-60), as primary small bowel carcinomas are usually rare. Although such data showed good en bloc resection rates (>70–80%), perforation rate was quite high (>30%) and some patients had delayed perforation, which required surgery. Moreover, ESD showed no differences in terms of long-term outcomes and survival when compared to EMR (58). For these reasons, ESGE does not recommend ESD for the treatment of superficial duodenal lesions, which are preferably treated with EMR and EPMR. Indeed, these techniques have a good safety profile and local recurrence is not that frequent (58,61). However, the majority of duodenal lesions included in the aforesaid studies were adenomas with very few cases of intramucosal and submucosal adenocarcinomas, and the risk of lymph node metastasis associated to such carcinomas has not been well described yet (17).
Technique
ESD consists of four steps, which are as follow: (I) margins delineation; (II) marking of the target lesion; (III) mucosal incision; (IV) submucosal dissection.
Margins delineation
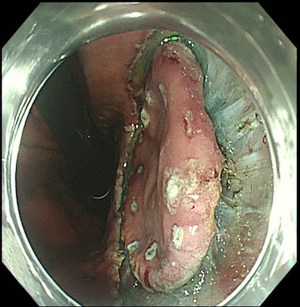
The first, very important step of upper GI ESD is represented by an accurate definition of lesion margins. As already stated in this paper, margins delineation can be achieved with chromoendoscopy, by using indigo carmine for gastric lesions, Lugol for SCC and acetic acid for BE associated EAC, or virtual chromoendoscopy (such as NBI, i-SCAN, FICE) with magnification (Figure 2). This step is essential in order to ensure the achievement of a complete resection with negative lateral margins.
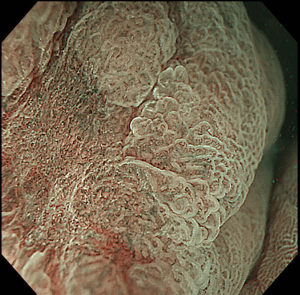
Marking of the target lesion
Prior to submucosal injection, the target lesion is marked either with an ESD needle knife or with argon plasma coagulation (Figure 3). This is done in order to recognize the edges of the lesion during the procedure, as they can be easily obscured by submucosal injection (62). The marking dots are usually placed at 5 mm from lesion margins (63). For EAC, marking dots can be placed up to 10 mm from lesion edges, as there can be subepithelial spread of the tumor (64). For SCC, marking is usually done close to the margins, in order to avoid stenosis from large dissection (64).
Mucosal incision
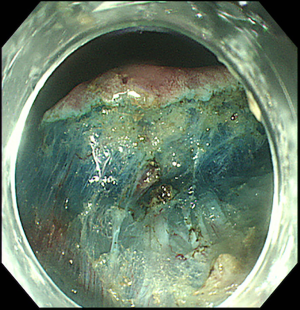
Prior to mucosal incision, the lesion is lifted with the injection of lifting agents, such as saline, 0.5% hyaluronate or glycerine, in order to create a submucosal fluid cushion (31,63). In particular, Gotoda et al. suggested the use of a mixture of normal saline, hydroxypropyl methylcellulose and indigo carmine (63), which has been shown to be safe when use for EMR (65). Once the lesion has been lifted, mucosal incision can be performed to expose the submucosal layer. For EGC the incision is made circumferentially at about 5 mm from the marking dots, in order to leave at least 10 mm of normal tissue between the incision and the tumor (64) (Figure 4). For esophageal tumors a partial incision is preferred, in order to avoid fluid escape from the submucosal layer and, therefore, to obtain a lasting submucosal lift and a safer dissection (64). With respect to the incision depth, there is no clear standard but it has been suggested that it should be done just beneath the muscularis mucosae (66).
Submucosal dissection
ESD technique may vary upon endoscopists, devices used and degree of fibrosis, but there are some general rules, which should be always respected. The exposed submucosal layer is expanded with the injection of the lifting solution first (usually normal saline). The preferred site for reinjection is usually just above the proper muscular layer (63). By using the distal attachment as a retractor, the endoscope is then advanced into the submucosal space below the lesion. Once the endoscope has entered the submucosal space, the ESD knife can be pulled out and the submucosal plane is then dissected parallel to the muscular layer (Figure 5). A wide range of ESD knives is available, some of them including the water jet function. The two traditional types are needle knives and insulated tip knives (Table 3).

Full table
Similarly to mucosal incision, there is no clear standard about the depth of dissection, but it has been suggested that it should be kept at the lower third of the submucosal layer, just beneath the vascular network and above the muscular layer (66). In order to maintain an adequate depth of dissection during the procedure, it is of main importance to understand whether the blood vessels exposed are horizontal small branches of the vascular network or large branches of the penetrating vessels. In the first case, the dissection should be performed below the horizontal small branches, while in case of large branches of the penetrating vessels, the dissection at the base of the vessels should be performed after their pre-coagulation with the ESD knife or haemostatic forceps (66). The density and thickness of blood vessels vary upon the GI segment. Indeed, in the gastric antrum and lesser curvature such density is low, thus the dissection is usually clear and quite easy. On the contrary, in the greater curvature, anterior/posterior wall of the stomach and esophagus, the density of submucosal vessels is high; therefore the dissection must be carried out very carefully in order to minimize intraoperative bleeding (66). Once the blood vessels have been dissected, submucosal dissection can be carried on in order to form a mucosal flap and to gradually and safely remove the lesion.
When submucosal fibrosis is found, ESD becomes more challenging, even for skilled endoscopists (67-69). Indeed, submucosal fibrosis, which is usually associated to tumor size, location, ulceration, histology type and tumor invasion (70), makes the lifting of the lesion difficult and leads to a higher risk of complications, such as perforation and intraprocedural bleeding (10,69). Therefore, to safely dissect fibrotic tumors, the procedure should start where there is as little fibrosis as possible, in order to form the mucosal flap (66); dissection should be then directed toward the most fibrotic area. Moreover, submucosal dissection should be carried on little by little (1 or 2 mm at a time), instead of dissecting in a continuous way (66) and sodium hyaluronate should be used for submucosal injection instead of normal saline, to obtain a long-lasting lifting (71).
Once dissection is completed, visible blood vessels should be coagulated, as post-procedural bleeding is one of ESD major complications, especially in the stomach (72). Coagulation of pulsating visible vessels should be minimized instead, in order to prevent postoperative perforation related to transmural thermal injury of the muscular layer (66).
Complications
The most common ESD-related complications are bleeding, perforation and stricture. Most of them can be managed endoscopically (73).
Bleeding
Most bleeding occurs during the procedure or shortly after that and can be usually controlled by electrocautery (73,74). Both immediate and delayed bleedings are more common in gastric ESD, because of the higher density of blood vessels in the stomach compared to the esophagus. The incidence of bleeding after gastric ESD has been reported to range between 1.8% and 15.6% (10,75-77).
Bleeding can be partially prevented by coagulation of the visible vessels on the ESD ulcer and by proton pump inhibitor therapy (78,79).
Perforation
Perforation rates vary upon the location, being higher for duodenal ESD (>30%) (58-60) compared to esophageal (up to 6%) and gastric ESD (1.2–5.2%) (73,80). Such complication can be managed endoscopically (with the use of hemoclips or any other closure device) most of the time, but some cases may require surgery (81), especially when perforation occurs in the esophagus or duodenum (58-60).
Stricture
Stricture formation mainly occurs after esophageal ESD and its rate ranges from 2% to 26% (14). The risk increases in case of long segment removal or circumferential dissection (14). As already mentioned before, esophageal stricture formation can be prevented with oral or intralesional steroids administration (20,21). Gastric stricture is less common, but it may occur after ESD, especially in the cardia and pre-pyloric area (82). Risk factors for gastric stenosis are a mucosal defect with a circumferential extent >3/4 and/or longitudinal extent >50 mm (82).
The majority of ESD-related strictures can be managed with endoscopic dilation (64).
Endoscopic surveillance
Scheduled endoscopic surveillance after curative ESD is always recommended, given the risk of synchronous and metachronous lesions. So far, there are no data in literature about the optimal surveillance schedule but ESGE suggests a first endoscopy at 3–6 months after ESD and then annually (17). Japanese guidelines also recommend annual or biannual endoscopy in all patients undergone ESD as well as a CT scan of the abdomen in those patients treated under expanded indications (11). With respect to the duration of endoscopic surveillance, although it may vary according to the individual risk of developing metachronous lesions, it should be continued indefinitely (83).
Conclusions
ESD is an innovative endoscopic treatment option for superficial GI lesions, as it allows their en bloc removal regardless of the shape and size. In Western countries, the procedure should be performed only in referral centers, as it requires a specific training and the acquisition of high-level technical skills. When deciding whether an upper GI lesion is eligible or not for ESD, many different factors should be taken into account, such as lesion features (especially lymph node involvement), patient’s condition and comorbidities, endoscopist’s experience and expertise and hospital’s resources (84). Therefore, indications and guidelines should be carefully followed in order to choose the best treatment in selected patients.
Acknowledgements
We want to thank Dr. Seiichiro Abe from National Cancer Center (Tokyo, Japan), who provided us with the figures presented in this paper.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and Oncologic Outcomes of Endoscopic Resection for Superficial Esophageal Neoplasm. Gut Liver 2015;9:470-7. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010;24:343-52. [Crossref] [PubMed]
- Seo GJ, Sohn DK, Han KS, et al. Recurrence after endoscopic piecemeal mucosal resection for large sessile colorectal polyps. World J Gastroenterol 2010;16:2806-11. [Crossref] [PubMed]
- Briedigkeit A, Sultanie O, Sido B, et al. Endoscopic mucosal resection of colorectal adenomas > 20 mm: Risk factors for recurrence. World J Gastrointest Endosc 2016;8:276-81. [Crossref] [PubMed]
- Toyonaga T, Man-I M, Morita Y, et al. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc 2009;21 Suppl 1:S31-7. [Crossref] [PubMed]
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015;81:583-95. [Crossref] [PubMed]
- Yahagi N, Fujishiro M, Omata M. Endoscopic submucosal dissection of colorectal lesion. Dig Endosc 2004;16:S178-81. [Crossref]
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010;72:1217-25. [Crossref] [PubMed]
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009;69:1228-35. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005;6:119-21. [Crossref] [PubMed]
- Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015;20:207-39. [Crossref] [PubMed]
- Mejía-Pérez LK, Abe S, Stevens T, et al. A minimally invasive treatment for early GI cancers. Cleve Clin J Med 2017;84:707-17. [Crossref] [PubMed]
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011;25:2541-6. [Crossref] [PubMed]
- Cao Y, Liao C, Tan A, et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751-7. [Crossref] [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009;6:1-25.
- Katada C, Muto M, Manabe T, et al. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003;57:165-9. [Crossref] [PubMed]
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011;73:1115-21. [Crossref] [PubMed]
- Hanaoka N, Ishihara R, Takeuchi Y, et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy 2012;44:1007-11. [Crossref] [PubMed]
- Neuhaus H, Terheggen G, Rutz EM, et al. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett's esophagus. Endoscopy 2012;44:1105-13. [Crossref] [PubMed]
- Chevaux JB, Piessevaux H, Jouret-Mourin A, et al. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett's neoplasia. Endoscopy 2015;47:103-12. [PubMed]
- Probst A, Aust D, Märkl B, et al. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015;47:113-21. [PubMed]
- Alvarez Herrero L, Pouw RE, van Vilsteren FG, et al. Safety and efficacy of multiband mucosectomy in 1060 resections in Barrett's esophagus. Endoscopy 2011;43:177-83. [Crossref] [PubMed]
- Thomas T, Gilbert D, Kaye PV, et al. High-resolution endoscopy and endoscopic ultrasound for evaluation of early neoplasia in Barrett's esophagus. Surg Endosc 2010;24:1110-6. [Crossref] [PubMed]
- Endoscopic Classification Review Group. Update on the Paris Classification of Superficial Neoplastic Lesions in the Digestive Tract. Endoscopy 2005;37:570-8. [Crossref] [PubMed]
- Takenaka R, Kawahara Y, Okada H, et al. Narrow-band imaging provides reliable screening for esophageal malignancy in patients with head and neck cancers. Am J Gastroenterol 2009;104:2942-8. [Crossref] [PubMed]
- Mannath J, Subramanian V, Hawkey CJ, et al. Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett's esophagus: a meta-analysis. Endoscopy 2010;42:351-9. [Crossref] [PubMed]
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015;47:122-8. [Crossref] [PubMed]
- Stahl M, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi51-6. [Crossref] [PubMed]
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett's adenocarcinoma. Gastrointest Endosc 2014;80:239-45. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009;70:860-6. [Crossref] [PubMed]
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015;12:355-61. [PubMed]
- Nakamura K, Ueyama T, Yao T, et al. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer 1992;70:1030-7. [Crossref] [PubMed]
- Shimizu S, Tada M, Kawai K. Early gastric cancer: its surveillance and natural course. Endoscopy 1995;27:27-31. [Crossref] [PubMed]
- Takekoshi T, Baba Y, Ota H, et al. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy 1994;26:352-8. [Crossref] [PubMed]
- Hiki Y, Shimao H, Mieno H, et al. Modified treatment of early gastric cancer: evaluation of endoscopic treatment of early gastric cancers with respect to treatment indication groups. World J Surg 1995;19:517-22. [Crossref] [PubMed]
- Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol 2005;3:S71-3. [Crossref] [PubMed]
- Takeuchi Y, Uedo N, Iishi H, et al. Endoscopic submucosal dissection with insulated-tip knife for large mucosal early gastric cancer: a feasibility study (with videos). Gastrointest Endosc 2007;66:186-93. [Crossref] [PubMed]
- Lian J, Chen S, Zhang Y, et al. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc 2012;76:763-70. [Crossref] [PubMed]
- Park YM, Cho E, Kang HY, et al. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc 2011;25:2666-77. [Crossref] [PubMed]
- Gotoda T, Iwasaki M, Kusano C, et al. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg 2010;97:868-71. [Crossref] [PubMed]
- Yamao T, Shirao K, Ono H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer 1996;77:602-6. [Crossref] [PubMed]
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999;125:148-54. [Crossref] [PubMed]
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-25. [Crossref] [PubMed]
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009;12:148-52. [Crossref] [PubMed]
- Nagahama T, Yao K, Maki S, et al. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc 2011;74:1259-67. [Crossref] [PubMed]
- Ninomiya Y, Yanagisawa A, Kato Y, et al. Unrecognizable intramucosal spread of diffuse-type mucosal gastric carcinomas of less than 20mm in size. Endoscopy 2000;32:604-8. [Crossref] [PubMed]
- Yao K, Oishi T, Matsui T, et al. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc 2002;56:279-84. [Crossref] [PubMed]
- Nakayoshi T, Tajiri H, Matsuda K, et al. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy 2004;36:1080-4. [Crossref] [PubMed]
- Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc 2016;28:3-15. [Crossref] [PubMed]
- Son SY, Park JY, Ryu KW, et al. The risk factors for lymph node metastasis in early gastric cancer patients who underwent endoscopic resection: is the minimal lymph node dissection applicable? A retrospective study. Surg Endosc 2013;27:3247-53. [Crossref] [PubMed]
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011;74:485-93. [Crossref] [PubMed]
- Chiu PW, Teoh AY, To KF, et al. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc 2012;26:3584-91. [Crossref] [PubMed]
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016;19:198-205. [Crossref] [PubMed]
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015;18:188-92. [Crossref] [PubMed]
- Nonaka S, Oda I, Tada K, et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy 2015;47:129-35. [PubMed]
- Matsumoto S, Miyatani H, Yoshida Y. Endoscopic submucosal dissection for duodenal tumors: a single-center experience. Endoscopy 2013;45:136-7. [PubMed]
- Jung JH, Choi KD, Ahn JY, et al. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy 2013;45:133-5. [Crossref] [PubMed]
- Alexander S, Bourke MJ, Williams SJ, et al. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc 2009;69:66-73. [Crossref] [PubMed]
- Sano T, Sasako M, Kinoshita T, et al. Recurrence of early gastric cancer. Follow-up of 1475 patients and review of the Japanese literature. Cancer 1993;72:3174-8. [Crossref] [PubMed]
- Gotoda T, Ho KY, Soetikno R, et al. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin N Am 2014;24:213-33. [Crossref] [PubMed]
- Bhatt A, Abe S, Kumaravel A, et al. Indications and Techniques for Endoscopic Submucosal Dissection. Am J Gastroenterol 2015;110:784-91. [Crossref] [PubMed]
- Bacani CJ, Woodward TA, Raimondo M, et al. The safety and efficacy in humans of endoscopic mucosal resection with hydroxypropyl methylcellulose as compared with normal saline. Surg Endosc 2008;22:2401-6. [Crossref] [PubMed]
- Toyonaga T, Nishino E, Man-I M, et al. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc 2012;45:362-74. [Crossref] [PubMed]
- Imagawa A, Okada H, Kawahara Y, et al. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy 2006;38:987-90. [Crossref] [PubMed]
- Kim ES, Cho KB, Park KS, et al. Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy 2011;43:573-8. [Crossref] [PubMed]
- Ahn JY, Choi KD, Choi JY, et al. Procedure time of endoscopic submucosal dissection according to the size and location of early gastric cancers: analysis of 916 dissections performed by 4 experts. Gastrointest Endosc 2011;73:911-6. [Crossref] [PubMed]
- Jeong JY, Oh YH, Yu YH, et al. Does submucosal fibrosis affect the results of endoscopic submucosal dissection of early gastric tumors? Gastrointest Endosc 2012;76:59-66. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Nakamura M, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc 2006;63:243-9. [Crossref] [PubMed]
- Toyonaga T, Man-I M, Fujita T, et al. The performance of a novel ball-tipped Flush knife for endoscopic submucosal dissection: a case-control study. Aliment Pharmacol Ther 2010;32:908-15. [Crossref] [PubMed]
- Oda I, Suzuki H, Nonaka S, et al. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013;25 Suppl 1:71-8. [Crossref] [PubMed]
- Kothari S, Kaul V. Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection for Endoscopic Therapy of Barrett's Esophagus-related Neoplasia. Gastroenterol Clin North Am 2015;44:317-35. [Crossref] [PubMed]
- Goto O, Fujishiro M, Oda I, et al. A multicenter survey of the management after gastric endoscopic submucosal dissection related to postoperative bleeding. Dig Dis Sci 2012;57:435-9. [Crossref] [PubMed]
- Lim JH, Kim SG, Kim JW, et al. Do antiplatelets increase the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms? Gastrointest Endosc 2012;75:719-27. [Crossref] [PubMed]
- Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 2009;58:331-6. [Crossref] [PubMed]
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy 2008;40:179-83. [Crossref] [PubMed]
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007;102:1610-6. [Crossref] [PubMed]
- Ono S, Fujishiro M, Koike K. Endoscopic submucosal dissection for superficial esophageal neoplasms. World J Gastrointest Endosc 2012;4:162-6. [Crossref] [PubMed]
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015;21:12635-43. [Crossref] [PubMed]
- Coda S, Oda I, Gotoda T, et al. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009;41:421-6. [Crossref] [PubMed]
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015;47:1113-8. [Crossref] [PubMed]
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: Systematic review. World J Gastroenterol 2015;21:13177-87. [Crossref] [PubMed]