Minimally invasive versus conventional spine surgery for vertebral metastases: a systematic review of the evidence
Introduction
Each year more than 1.5 million Americans are diagnosed with some form of cancer (1) and 40–70% of these patients will develop vertebral metastases (2-7). This makes for upwards of 1 million Americans annually who are diagnosed with vertebral metastases. These lesions may develop through direct extension, lymphatic spread, dissemination through nutrient arteries, or most commonly through Batson’s venous plexus (8,9). Any tumor has the potential to seed the vertebral column, but spinal metastases are most common secondary to lung (24% of cases), breast (24%), liver (12%), prostate (11%), and kidney (11%) primary tumors (10-22). Though the majority of these lesions are subclinical, as much as 10% of patients may present with symptoms of mechanical instability or epidural cord compression (10,23,24), including weakness, sensory disturbances, bowel or bladder dysfunction, and gait disturbance.
Treatment of spinal metastases is dependent upon the patient’s clinical pictures and expected survival, as well as the location and focality of the spinal tumor (25). In the overwhelming majority of cases, the symptomatic spinal metastasis represents just one of many sites of disease. Consequently, the goal of surgery is not cure, but rather palliation (12,13,18,21,22,25-33). As patients are being considered for surgery, one of the key selection criteria is expected patient survival. Multiple prognostic scoring systems have been developed to aid providers in determining post-operative live expectancy, including the Tokuhashi (34,35), Sioutos (18), van der Linden (31), Tomita (20), and Bauer scales (36,37). In general, guidelines suggest that patients with life expectancies less than 3 months be treated non-surgically (19,21,30,38-46), as the procedure-associated morbidity outweighs the potential therapeutic benefits. However, new advances in minimally invasive (MIS) surgical approaches promise to reduce the morbidity associated with the surgical treatment of spinal metastases. Consequently, surgery may become an option in patients for whom conventional approaches are deemed too morbid. The goals of this review are to: (I) report the extant literature directly comparing MIS and conventional approaches to the operative management of spinal metastases; and (II) perform a meta-analysis of the results.
Methods
Search strategy
A systematic review of the English literature available on PubMed was performed, along with a review of the bibliographies of the examined articles. The query utilized in the PubMed search was designed to include as many articles as possible pertaining to the pathology and interventions of interest. The final search string was: (minimally invasive surgery OR MIS OR MISS OR VAST OR mini-open spine surgery OR endoscopic thoracoscopy) AND (spine OR vertebra OR vertebrae OR spinal) AND (metastasis OR bone neoplasm OR bone tumor OR spine neoplasm OR spine tumor OR metastatic epidural spinal cord compression OR MESCC OR ESCC OR spinal instability)
Eligibility criteria
- Criteria used for the inclusion of articles were:
- Articles published prior to October 1, 2017;
- Full-text availability in English or full-English translation;
- Article reports surgical treatment of vertebral metastases or epidural metastases;
- Adult population (all patients ≥18 years old);
- Articles directly compared a MIS technique with a conventional approach;
- Article reports clinical and intra-operative results for both conventional and MIS cohorts;
- Format of the article is a randomized controlled trial, nonrandomized trial, case series (≥ two patients), case-control study, or cohort study;
- Article is prospective or retrospective;
- Metastases involve mobile spine (C1-L5).
- Criteria used for exclusion of articles were:
- Article reports primary spinal tumors;
- Article reports intradural tumors;
- Article reports outcomes of stereotactic radiosurgery, vertebroplasty, or kyphoplasty;
- Article reports on patients with lumbosacral pathology;
- Study population includes patients less than 18 years of age;
- Article fails to report intra-operative and quantifiable clinical data for both approaches (study either pools results or performs only qualitative analysis of results).
Study eligibility
Abstracts were screened by two reviewers (Z Pennington and AK Ahmed) using the inclusion and exclusion criteria stated above. In cases of disagreement, a third review (CA Molina) was involved to make the final decision. Full-text versions of articles meeting the criteria were gathered and reviewed in full to determine eligibility for inclusion in the final analysis. Data relevant to the questions of this review were then extracted and tabulated. The inclusion and exclusion of studies was performed according to the latest version of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (www.prisma-statement.org).
Results
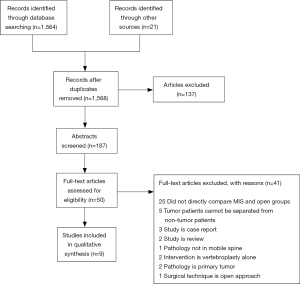
The results of our search are summarized in the PRISMA flow diagram shown in Figure 1. In brief, our search returned 1,564 records from the PubMed database; an additional 21 records were identified through other sources, which led to a total of 1,568 articles after elimination of duplicates. The abstracts of 187 articles were screened based upon their title, of which 137 were excluded. Full-text versions of the remaining 50 studies were gathered and screened for further eligibility. Of these 50 studies, 9 studies were found to meet inclusion criteria for the study and were included in the qualitative analysis. Of the 41 excluded articles, 25 were excluded because they did not directly compare MIS and open groups, 5 were excluded because the data for the tumor patients could not be separated from those with other pathologies, 3 studies were found to be case reports, 2 studies were review articles, 2 studies investigated vertebroplasty-alone, 2 studies examined only primary tumors, 1 study reported pathology of the sacrum only, and 1 study reported on the use of technique equivalent to conventional open approaches.
The main outcomes we extracted from the reports included the MIS technique utilized, mean blood loss, operating time, hospital length of stay, prevalence and extent of neurological improvement, complication rate, and degree of pain palliation. Additionally, for articles directly comparing open and MIS techniques, we also extracted inferential statistics comparing the outcomes of the two groups with respect to blood loss, operative time, length of stay, complication rate, and neurological recovery. For classification of the level of evidence, we utilized the “Levels of Evidence for Primary Research Question” adopted by the North American Spine Society.
Direct comparisons of MIS and conventional techniques
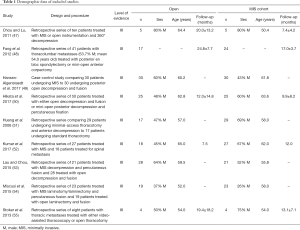
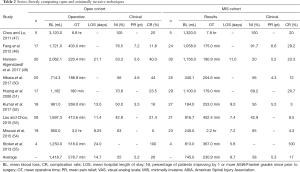
Our search criteria yielded nine studies directly comparing MIS and open approaches for the treatment of unstable or symptomatic vertebral metastases (Tables 1,2). Seven of the nine studies are retrospective case series of patients operated with minimally-invasive or open techniques, one study is a prospective case series, and one study, that by Hansen-Algenstaedt et al., is case-control study directly comparing the two classes of treatments. All results were classified as level III evidence and reported between 8 and 60 total patients. The earliest of these comparative studies was reported by Huang and colleagues in 2006 (51). These authors reported a retrospective series of 29 patients (68.9% male; mean 58 years old) undergoing minimal access thoracotomy and anterior decompression and 17 patients (47.1% male; mean 57 years old) undergoing standard thoracotomy. In the MIS cohort, mean blood loss was 1,100 mL (200–4,300 mL) and mean operative time was 179 minutes (120–250 minutes). Sixty-nine percent of patients improved by one or more Frankel grades and complications were seen in 20.7% of patients. The open cohort had statistically similar outcomes, with a mean blood loss of 1,162 mL (300–3,000 mL), a mean operative time of 180 minutes (120–315 minutes), neurological improvement ≥1 Frankel grade in 70.8% of patients, and complications in 23.5% of patients. The groups were similar for all endpoints examined except the need for post-operative intensive care unit (ICU) stay; only 6.9% of the minimal access group required a post-operative ICU stay compared to 88% of the open cohort (P<0.0001).
Full table
Full table
The next study comparing MIS and open techniques was published by Chou and Lu, who reported a series of 16 patients treated with either MIS posterior decompression or corpectomy with transfascial instrumentation, or open decompression and fusion (47). Within each cohort, five patients were treated for spinal metastases. Mean blood loss within the MIS cohort was 1,320 mL (600–2,000 mL) and mean operative time was 6.8 hours (6–9 hours). Within the open cohort, mean blood loss was 3,120 mL (350–7,800 mL) and mean operative time was 6.8 hours (5–7 hours). Both groups saw motor performance improve to 4+/5 or better in all patients post-operatively and complications in 20% of patients, with one wound infection in the MIS group and one epidural hematoma in the open group. Neither pain improvement nor length of stay was reported for either group.
In 2012, Fang and colleagues reported a series of 41 patients undergoing surgery for thoracolumbar metastases, who were treated with either posterior en bloc spondylectomy (n=17; 41.2% female; mean 51.0 years old) or mini-open anterior corpectomy (n=24; 62.5% female; mean 56.6 years old) (48). Indications for surgery were intractable pain, acute, progressive neurological deficits, or spinal instability. Within the open cohort, mean blood loss was 1,721±293 mL and mean operative time was 403±55 minutes, which were significantly greater (P=0.0001) than the blood loss (1,058±263 mL) and operative duration (175±38 minutes) seen in the MIS cohort. The degree of neurological improvement was statistically similar between groups (P=0.063), with 91.7% of patients in the mini-open group improving one or more American Spinal Injury Association (ASIA) grades, and 76.5% of patients in the open cohort improving by at least one ASIA grade. Pain relief was also similar between groups, both of which showed significant improvements in visual analogue scale (VAS) pain score relative to pre-operative pain status—open (6.6 points; P=0.000) and MIS (6.4 points; P=0.000). Complications were more common in the open group (29.2%) than in the MIS group (11.8%), though this difference did not prove statistically significant. Recurrence was more common in the MIS group (20.8% vs. 0.0%; P=0.045), but this did not contribute to a significant survival difference between the groups.
Stoker et al. published a series of eight patients (62.5% male; mean 54.3 years old) treated with either video-assisted thoracoscopy (VATS) or open thoracotomy (OT) for symptomatic thoracic metastases secondary to non-small cell lung cancer (55). Patients in body groups were grossly similar with respect to age at surgery (VATS: 54 vs. OT: 54), BMI (24 vs. 22 kg/m2), cigarette smoking history (44 vs. 36 pack-years), and tumor size (7.3 vs. 7.3 cm), and all patients were Eastern Cooperative Oncology Group (ECOG) 0 or 1. Patients in the VATS group experienced lower blood loss (813±463 vs. 1,250±1,500 mL) and operative time (367±117 vs. 518±264 minutes) than those treated with OT. Intraoperative complications were documented in one patient from each group and perioperative and post-operative complications were documented in all patients in both groups. Mean length of stay was much lower for the VATS group (5.8 days) than the OT group (24 days). Neither neurological improvement nor pain relief was quantified by the authors.
In 2015, Lau and Chou published a series of 49 patients treated for symptomatic vertebral metastases, including the 10 patients previously reported by Chou and Lu (53). Their series compared 21 patients (52.4% male; mean 55.8 years old) undergoing MIS transpedicular corpectomy, posterior decompression, and transfascial instrumentation to 28 patients (64.3% male; mean 59.5 years old) undergoing open decompression and fusion. Patients in the mini-open group had significantly lower blood loss (916.7 vs. 1,697.3 mL; P=0.019) and length of stay (7.4 vs. 11.4 days; P=0.001) than those in the open cohort. The two groups were similar though with regards to operative time (452.4 vs. 413.6 minutes; P=0.329), percentage of patients requiring transfusion (57.1% vs. 64.3%; P=0.611), perioperative complication rate (9.5% vs. 21.4%; P=0.265), number of fused segments (P=0.292), and number of levels corpectomized (P=0.413). Post-operatively, 42.9% of patients in each group improved by 1 or more ASIA grades. One patient in the mini-open cohort and two patients in the open cohort required a revision operation (P=0.803) and post-operative complications were seen in 14.3% of patients in each group.
Miscusi and colleagues reported a retrospective series of 41 patients treated for thoracic metastases causing acute myelopathy (54). Patients were treated with either MIS laminotomy/laminectomy with percutaneous fusion (n=23; 73.9% female; mean 58 years old) or open laminectomy and fusion (n=19; 63.2% female; 52 years old). Compared to the open group, patients in the MIS group had significantly shorter operative times (2.2 vs. 3.2 hours; P<0.01), lower blood loss (240 vs. 900 mL; P<0.01), lower likelihood of requiring blood transfusion (0% vs. 63.2%; P<0.01), shorter post-operative bed rest (2 vs. 4 days; P<0.01), and shorter hospital length of stay (7.2 vs. 9.25 days; P<0.01). The prevalence of complications did not differ significantly between the two groups, however, 4.3% in the MIS group vs. 0% in the open group (P>0.05). Both groups showed significant improvement in neurological status, with 65% of patients in the MIS group and 63% of patients in the open group reported a neurological improvement of ≥1 ASIA grade. Patients in the MIS cohort were more likely to report an improvement in pain as measured by the VAS pain scale though (74% vs. 53%; P=0.007), and reported greater improvements in quality of life, symptoms, and functional outcome scores as measured on the European Organization for Research and Treatment of Cancer QOL questionnaire (EORTC QLQ-C30) and Bone Metastasis module (EORTC QLQ-BM22).
The only case-control study published to date comparing open and MIS techniques was reported by Hansen-Algenstaedt and colleagues (49). The authors compared 30 patients undergoing open posterior decompression and fusion (60% male; mean 60.2 years old) to 30 patients (43.3% male; mean 61.8 years old) undergoing percutaneous pedicle screw fixation with some form of mini-open or thoracoscopic decompression, the nature of which was dictated by the level of the lesion and location within the affected vertebra. Both cohorts were statistically similar with regards to ASA score, Frankel grade, tumor type, demographics, and pre-operative treatment. Patients in the MIS group had lower blood loss (1,156.0±572.3 vs. 2,062.1±1,148.0 mL; P<0.001), fewer patients requiring blood transfusion (40% vs. 76.7%; P=0.006), and shorter hospital stays (11.0±5.0 vs. 21.1±10.8 days; P<0.001). The MIS group did have a larger number of instrumented segments on average (5.5±3.1 vs. 3.8±1.7; P=0.012), but had fewer patients requiring posterior decompression (66.7% vs. 96.7%; P=0.003), fewer decompressed segments (1.0±1.0 vs. 1.8±0.8 levels; P=0.001) and longer fluoroscopy time (116.1±63.3 vs. 69.9±42.6 s; P=0.002). Post-operative outcomes were grossly similar between the two groups. In the MIS group, 20% of patients improved by 1 or more Frankel grades (P=0.014), mean VAS pain score improvement was 2.0 points at post-op day 7 and 5.2 points at 3-month follow-up, improvement in ECOG score was 0.6 at 3-month follow-up (P=0.003), and improvement in Karnofsky performance status (KPS) was 10% at 3-month follow-up (P<0.001). In the open cohort, 33.3% of patients improved by one or more Frankel grades (P=0.006), mean pain improvement was 3.1 points at post-op day 7 and 4.6 points at 3-month follow-up, mean ECOG improvement was 1.0 at 3-month follow-up (P<0.001), and mean KPS score improvement was 20% at 3-month follow-up (P<0.001).
Kumar et al. published a prospective series comparing 27 patients (67% male; mean 62 years old) treated with percutaneous pedicle screw fixation with or without mini-open posterior decompression and 18 patients (45% male; mean 65 years old) treated with open decompression and fusion (52). The majority of patients had gross or potentially unstable spines with 100% of the MIS group and 95% of the open group having a Spinal Instability Neoplastic Score (SINS) of 7 or more. Compared to patients in the open cohort, those undergoing MIS management had significantly lower blood loss (184 vs. 961 mL; P<0.001) and shorter time to the start of radiotherapy (13 vs. 24 days; P<0.001). No differences were reported with regards to operative time (MIS: 253 minutes vs. Open: 269 minutes), duration of ICU stay (MIS: 1 day vs. Open: 1 day), or duration of hospital stay (MIS: 9 days vs. Open: 13 days). Both groups showed significant improvement in VAS pain scores post-operatively; patients in the MIS cohort had a mean improvement of 5.2 points (P<0.001) and those in the open cohort had a mean improvement of 3.5 points (P<0.001). Neurological improvement greater than 1 Frankel grade was seen in 56% of patients in the MIS cohort and 50.0% of patients in the open cohort. Functional improvement was also significant for all patients, with a mean ECOG improvement of 0.5 in the MIS group (P<0.001) and 0.6 for the open group (P<0.001). In both groups the majority of patients were ambulatory at 3-month follow-up (MIS: 79% vs. open: 64%), but the authors did not report the percentage of patients who recovered ambulation following surgery. Lastly, median survival was longer in the open cohort (12 vs. 7.5 months), but this difference was not reported as significant and 3-month overall survival rates were the same for both groups (MIS: 74% vs. open: 75%).
The most recent comparison of open and MIS procedures was reported by Hikata and colleagues, who published a retrospective series of 50 patients (54% male; mean 63.2 years old) treated with either mini-open posterior decompression and percutaneous pedicle screw fixation (n=25) or open decompression and fusion (n=25) (50). Pre-operatively both groups were grossly similar in terms of age, sex, spinal level, KPS, diffusivity of spinous metastatic disease, presence of visceral metastases, Frankel grade, and Tokuhashi score. MIS patients were reported to have significantly lower blood loss (340.1±302.5 vs. 724.3±545.9 mL; P=0.005), to be significantly less likely to require red blood cell (RBC) transfusion (12% vs. 40%; P=0.029), and to require significantly shorter post-operative bed rest (2.0±1.5 vs. 3.6±1.6 days; P<0.001). The two groups were otherwise similar with respect to operative time (MIS: 204.6±55.4 minutes vs. Open: 188.9±43.6 minutes) and the number of levels fused (MIS: 5.1±1.3 vs. Open: 5.9±2.3). Both groups also provided statistically similar levels of neurological improvement—56% of the MIS cohort and 56% of the open cohort improved 1 or more Frankel grades—and pain improvement on the VAS scale (MIS: 4.6±3.0 vs. Open: 5.0±3.0). Complications were less common in the MIS group though, with only 12% of MIS patients experiencing a complication, compared to 44% of patients in the open cohort (P=0.012).
Summary
We identified nine studies directly comparing the results of MIS (n=183 unique patients) and open procedures (n=163 unique patients). Averaging across all studies, mean operative blood loss in the open cohort is 1,418.7 mL (range, 714.3–3,120 mL), mean operative time is 278.7 minutes (range, 180–518 minutes), and mean hospital length of stay is 14.7 days (range, 9.25–24 days). Average pain improvement in the open cohort was 5.2 points on the VAS scale (range, 3.5–7.2), 55% of patients reported neurological improvement of one or more ASIA or Frankel grades (range, 33–100%), and complications were seen in 26% of patients (range, 0–100%). For the patients undergoing MIS approaches, mean blood loss was 745.0 mL (range, 184–1,320 mL), mean operative time was 230.9 minutes (range, 132–468 minutes), and mean hospital length of stay was 8.7 days (range, 5.8–11 days). Within this group of studies, 58% of MIS patients improved one or more ASIA or Frankel grades post-operatively (range, 20–100%), mean pain improvement was 5.3 points on the VAS scale (range, 4.3–6.6), and complications were reported in 17% of patients (range, 3–100%). Due to the high degree of heterogeneity in the MIS techniques reported by the different studies, as well as the inability to disentangle the results of different MIS approaches within several of the studies, it was not possible to perform a meta-analysis of the results. Qualitatively however, MIS operations were associated with lower blood loss (745.0 vs. 1,418.7 mL), shorter operative times (230.9 vs. 278.7 minutes), shorter hospital stays (8.7 vs. 14.7 days), and lower complication rates (17% vs. 26%), while providing similar neurological improvement (58% vs. 55%) and pain relief (5.3 vs. 5.2 points on VAS scale).
Case report
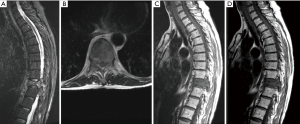
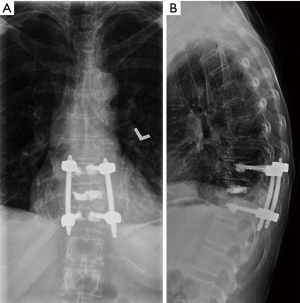
A 55-year-old female with a history of breast cancer presented to the clinic of IL with severe back pain that was mechanical in nature. The patient was evaluated radiographically and was found to have a lytic T10 lesion involving the vertebral body and both pedicles. The lesion was evaluated using the SINS (56) and Epidural Spinal Cord Compression (ESCC) Scale (57,58), which revealed a ESCC grade 1c lesion with a SINS score of 12, suggesting a potentially unstable segment (Figure 2). As a result, the patient was offered an MIS procedure with vertebroplasty of the lytic segment and short-segment percutaneous instrumentation (Figure 3).
Discussion
The treatment of spinal metastases is largely guided by a combination of how advanced is the patient’s disease and the severity of the symptoms displayed by the patient (25). For the overwhelming majority of patients with spinal metastases, the lesions remain asymptomatic, or the severity of symptoms is mild enough that patients can be treated with a combination of radiotherapy and systemic agents. However, in 14% or more of patients (24,30,59-63), patients present with neurological symptoms secondary to oncologic compression of neural elements or intractable pain secondary to spinal instability. In these cases, the preferred route of treatment is less obvious. Up until a decade ago, standard therapy for these patients was also a combination of radiation and systemic therapy, as this had proved equally effective at providing symptomatic relief, while at the same time having lower associated morbidity than contemporary surgical techniques. In 2005 though, Patchell and colleagues published the results of a trial of 101 patients randomized to either radiotherapy and surgical decompression (n=50) or radiotherapy alone (n=51) (30). Patients randomized to the former group were significantly more likely to ambulate post-operatively (OR =6.2; P=0.001), as well as demonstrate improvements in their ASIA motor score (P<0.006) and Frankel grade (P=0.0008) 30 days post-operatively. These results were supported by contemporaneous retrospective studies (5,45,64), and consequently surgery has become a standard consideration for all patients with symptomatic vertebral metastases.
Unlike surgery for primary spinal tumors, the goal of surgery for spinal metastases is overwhelming palliation of symptoms, not cure (12,13,18,21,22,25-33). As a result, the consulting surgeon must consider the patient’s overall health, as well as the clinical picture of the vertebral metastases. The majority of published studies have suggested that surgery only be considered in patients with a life expectancy greater than 3 to 6 months (19,21,30,38-46), as patients with lower life expectancies are liable to have complication profiles that far outweigh any potential benefit of surgery. Unfortunately, this means that many patients, especially those with metastases secondary to aggressive primary pathologies, such as lung cancer, pancreas cancer, and melanoma, are ineligible for surgical symptom palliation due to the aggressive course of their disease (22,26,28,29,33,38,65,66). In the past decade though, multiple groups have begun to publish the results of patients with degenerative spine pathologies treated with MIS techniques. These authors report MIS techniques to have lower associated soft tissue damage (67,68), lower post-operative infection rates (53,69-73), lower post-operative pain, and shorter hospital lengths of stay (74), while providing functional results similar to conventional procedures (45,54,67,75-77). Consequently, multiple authors have begun to explore the application of these techniques to patients with vertebral metastases, a medically-vulnerable population less able to deal with the rigors of conventional surgery.
In this review, we reported the results of nine studies directly comparing the results of MIS and open techniques for the treatment of symptomatic vertebral metastases. In total, these studies compared 183 patients treated with MIS techniques to 163 patients treated with conventional approaches to decompression and fusion. Six of the studies reported significantly lower blood loss in the MIS group (48-50,52-54), three reported significantly shorter operative times (48,49,54), four reported significantly shorter recovery times (50,52-54), two reported a lower complication rate (48,50), and four reported similar or superior improvements in pain post-operatively (48,49,52,54). Additionally, five studies reported the MIS techniques to provide clinically similar improvements in neurological function (48-50,52,54).
Together, these results suggest that MIS techniques might be an option for patients with vertebral metastases who are otherwise too unhealthy to undergo surgery, whether due to age, extent of systemic disease, presence of medical comorbidity, or low expected survival. These results must be carefully considered though, as all of the available evidence is low quality—nine of the studies are considered level III evidence and the remaining 25 studies are considered to be level IV evidence, comprising mostly retrospective case series. Nevertheless, MIS techniques show promise in the palliative management of spinal metastases, especially with the improvements being made in the field of stereotactic radiosurgery. Because of these advances, some centers are now moving towards utilizing “separation surgery” as their standard of care (78,79). In this treatment method, patients are first surgically decompressed and then treated with high dose, single fraction (18–24 Gy) or hypofractionated (18–30 Gy in 3–6 fractions) focal radiation (78-81). The aim of surgery in these cases is to create a resection cavity around the spinal cord at the lesion level, and then to address the remaining tumor using the stereotactic radiation. MIS surgery seems well-matched to this indication, as it decreases recovery time and so potentially allows for the faster delivery of radiation (82). Currently, there is no high-quality evidence to support this claim, but with the increased use of MIS techniques, such evidence may become available in the near future. Until such point, the choice to utilize MIS techniques is one that must be made by the patient and their attending surgeon. While the techniques have a steeper learning curve than traditional, open techniques, they have a lower associated morbidity and so may be the only reasonable option available to the sickest of patients.
Conclusions
Spinal metastases are a relatively common clinical phenomenon, affecting 40–70% of the more than 1,000,000 cancer patients diagnosed annually in the United States. As the disease course progresses, these lesions can produce intractable mechanical pain and spinal instability, or even neurological dysfunction secondary to direct compression of the neural elements. Unfortunately, these most advanced patients are also those who are least able to tolerate the morbidity associated with surgical decompression and fusion. Over the past decade though, MIS techniques have been developed, which current evidence suggests may provide similar improvements in neurological function and pain relief, while decreasing the morbidity of surgery, including blood loss, operative time, complication rate, and in-patient length of stay. The overall quality of evidence currently available is low—all evidence is currently class III or IV—and consequently, the decision to utilize MIS techniques is one that should be made based upon patient preference and surgeon familiarity.
Acknowledgements
None.
Footnote
Conflicts of Interest: AK Ahmed: Neurosurgery Research & Education Foundation Medical Student Summer Research fellow; CA Molina: consultant for Augmedics; I Laufer: consulting fees from BrainLab, DePuy/Synthes, SpineWave, Medtronic, and Globus; DM Sciubba: consultant for DePuy-Synthes, Medtronic, K2M, Globus, and Orthofix. The other authors have no conflicts of interest to declare.
References
- American Cancer Society. Cancer Facts & Figures 2017.
- Arguello F, Baggs RB, Duerst RE, et al. Pathogenesis of Vertebral Metastasis and Epidural Spinal Cord Compression. Cancer 1990;65:98-106. [Crossref] [PubMed]
- Fornasier VL, Horne JG. Metastases to the vertebral column. Cancer 1975;36:590-4. [Crossref] [PubMed]
- Gezercan Y, Çavuş G, Ökten AI, et al. Single-Stage Posterolateral Transpedicular Approach With 360-Degree Stabilization and Vertebrectomy in Primary and Metastatic Tumors of the Spine. World Neurosurg 2016;95:214-21. [Crossref] [PubMed]
- Klimo P Jr, Thompson CJ, Kestle JR, et al. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol 2005;7:64-76. [Crossref] [PubMed]
- Molina CA, Gokaslan ZL, Sciubba DM. A systematic review of the current role of minimally invasive spine surgery in the management of metastatic spine disease. Int J Surg Oncol 2011;2011:598148. [PubMed]
- Wiggins GC, Mirza S, Bellabarba C, et al. Perioperative complications with costotransversectomy and anterior approaches to thoracic and thoracolumbar tumors. Neurosurg Focus 2001;11:e4. [Crossref] [PubMed]
- Maccauro G, Spinelli MS, Mauro S, et al. Physiopathology of spine metastasis. Int J Surg Oncol 2011;2011:107969. [PubMed]
- Togawa D, Lewandrowski K. The Pathophysiology of Spinal Metastases. In: McLain RF, Markman M, Bukowski RM, et al. editors. Cancer in the Spine: Comprehensive Care. Totowa, NJ: Humana Press, 2006:17-23.
- Gokaslan ZL, York JE, Walsh G, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg 1998;89:599-609. [Crossref] [PubMed]
- Holman PJ, Suki D, McCutcheon IE, et al. Surgical management of metastatic disease of the lumbar spine: experience with 139 patients. J Neurosurg Spine 2005;2:550-63. [Crossref] [PubMed]
- Phanphaisarn A, Patumanond J, Settakorn J, et al. Prevalence and survival patterns of patients with bone metastasis from common cancers in Thailand. Asian Pac J Cancer Prev 2016;17:4335-40. [PubMed]
- Pointillart V, Vital J, Salmi R, et al. Survival prognostic factors and clinical outcomes in patients with spinal metastases. J Cancer Res Clin Oncol 2011;137:849-56. [Crossref] [PubMed]
- Quan GM, Vital J, Aurouer N, et al. Surgery improves pain, function and quality of life in patients with spinal metastases: a prospective study on 118 patients. Eur Spine J 2011;20:1970-8. [Crossref] [PubMed]
- Quraishi NA, Manoharan SR, Arealis G, et al. Accuracy of the revised Tokuhashi score in predicting survival in patients with metastatic spinal cord compression (MSCC). Eur Spine J 2013;22 Supp l:S21-6. [Crossref] [PubMed]
- Schaberg J, Gainor BJ. A profile of metastatic carcinoma of the spine. Spine (Phila Pa 1976) 1985;10:19-20. [Crossref] [PubMed]
- Schoenfeld AJ, Leonard DA, Saadat E, et al. Predictors of 30- and 90-Day Survival Following Surgical Intervention for Spinal Metastases: A Prognostic Study Conducted at Four Academic Centers. Spine (Phila Pa 1976) 2016;41:E503-9. [Crossref] [PubMed]
- Sioutos PJ, Arbit E, Meshulam CF, et al. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer 1995;76:1453-9. [Crossref] [PubMed]
- Tokuhashi Y, Ajiro Y, Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine (Phila Pa 1976) 2009;34:69-73. [Crossref] [PubMed]
- Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298-306. [Crossref] [PubMed]
- Yang SB, Cho W, Chang U. Analysis of prognostic factors relating to postoperative survival in spinal metastases. J Korean Neurosurg Soc 2012;51:127-34. [Crossref] [PubMed]
- Weigel B, Maghsudi M, Neumann C, et al. Surgical management of symptomatic spinal metastases. Postoperative outcome and quality of life. Spine (Phila Pa 1976) 1999;24:2240-6. [Crossref] [PubMed]
- Pinter NK, Pfiffner TJ, Mechtler LL. Neuroimaging of spine tumors. Handb Clin Neurol 2016;136:689-706. [Crossref] [PubMed]
- Ryken TC, Eichholz KM, Gerszten PC, et al. Evidence-based review of the surgical management of vertebral column metastatic disease. Neurosurg Focus 2003;15:E11. [Crossref] [PubMed]
- Sohn S, Kim J, Chung CK, et al. A nationwide epidemiological study of newly diagnosed spine metastasis in the adult Korean population. Spine J 2016;16:937-45. [Crossref] [PubMed]
- Arrigo RT, Kalanithi P, Cheng I, et al. Predictors of survival after surgical treatment of spinal metastasis. Neurosurgery 2011;68:674-81. [Crossref] [PubMed]
- Helweg-Larsen S, Sørensen PS, Kreiner S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys 2000;46:1163-9. [Crossref] [PubMed]
- Jansson KA, Bauer HC. Survival, complications and outcome in 282 patients operated for neurological deficit due to thoracic or lumbar spinal metastases. Eur Spine J 2006;15:196-202. [Crossref] [PubMed]
- North RB, LaRocca VR, Schwartz J, et al. Surgical management of spinal metastases: analysis of prognostic factors during a 10-year experience. J Neurosurg Spine 2005;2:564-73. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- van der Linden YM, Dijkstra SP, Vonk EJ, et al. Prediction of survival in patients with metastases in the spinal column. Results based on a randomized trial of radiotherapy. Cancer 2005;103:320-8. [Crossref] [PubMed]
- Vanek P, Bradac O, Trebicky F, et al. Influence of the Preoperative Neurological Status on Survival After the Surgical Treatment of Symptomatic Spinal Metastases With Spinal Cord Compression. Spine (Phila Pa 1976) 2015;40:1824-30. [PubMed]
- Wibmer C, Leithner A, Hofmann G, et al. Survival analysis of 254 patients after manifestation of spinal metastases: evaluation of seven preoperative scoring systems. Spine (Phila Pa 1976) 2011;36:1977-86. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 1990;15:1110-3. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Oda H, et al. A Revised Scoring System for Preoperative Evaluation of Metastatic Spine Tumor Prognosis. Spine (Phila Pa 1976) 2005;30:2186-91. [Crossref] [PubMed]
- Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand 1995;66:143-6. [Crossref] [PubMed]
- Leithner A, Radl R, Gruber G, et al. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J 2008;17:1488-95. [Crossref] [PubMed]
- Finkelstein JA, Zaveri G, Wai E, et al. A population-based study of surgery for spinal metastases. Survival rates and complications. J Bone Joint Surg Br 2003;85:1045-50. [Crossref] [PubMed]
- Hosono N, Ueda T, Tamura D, et al. Prognostic relevance of clinical symptoms in patients with spinal metastases. Clin Orthop Relat Res 2005.196-201. [Crossref] [PubMed]
- Kaloostian PE, Yurter A, Zadnik PL, et al. Current paradigms for metastatic spinal disease: an evidence-based review. Ann Surg Oncol 2014;21:248-62. [Crossref] [PubMed]
- Laufer I, Sciubba DM, Madera M, et al. Surgical management of metastatic spinal tumors. Cancer Control 2012;19:122-8. [Crossref] [PubMed]
- Quraishi NA, Gokaslan ZL, Boriani S. The surgical management of metastatic epidural compression of the spinal cord. J Bone Joint Surg Br 2010;92:1054-60. [Crossref] [PubMed]
- Sciubba DM, Gokaslan ZL, Suk I, et al. Positive and negative prognostic variables for patients undergoing spine surgery for metastatic breast disease. Eur Spine J 2007;16:1659-67. [Crossref] [PubMed]
- Sciubba DM, Goodwin CR, Yurter A, et al. A systematic review of clinical outcomes and prognostic factors for patients undergoing surgery for spinal metastases secondary to breast cancer. Global Spine J 2016;6:482-96. [Crossref] [PubMed]
- Smith ZA, Yang I, Gorgulho A, et al. Emerging techniques in the minimally invasive treatment and management of thoracic spine tumors. J Neurooncol 2012;107:443-55. [Crossref] [PubMed]
- Zadnik PL, Hwang L, Ju DG, et al. Prolonged survival following aggressive treatment for metastatic breast cancer in the spine. Clin Exp Metastasis 2014;31:47-55. [Crossref] [PubMed]
- Chou D, Lu DC. Mini-open transpedicular corpectomies with expandable cage reconstruction. Technical note. J Neurosurg Spine 2011;14:71-7. [Crossref] [PubMed]
- Fang T, Dong J, Zhou X, et al. Comparison of mini-open anterior corpectomy and posterior total en bloc spondylectomy for solitary metastases of the thoracolumbar spine. J Neurosurg Spine 2012;17:271-9. [Crossref] [PubMed]
- Hansen-Algenstaedt N, Kwan MK, Algenstaedt P, et al. Comparison Between Minimally Invasive Surgery and Conventional Open Surgery for Patients With Spinal Metastasis: A Prospective Propensity Score-Matched Study. Spine (Phila Pa 1976) 2017;42:789-97. [Crossref] [PubMed]
- Hikata T, Isogai N, Shiono Y, et al. A Retrospective Cohort Study Comparing the Safety and Efficacy of Minimally Invasive Versus Open Surgical Techniques in the Treatment of Spinal Metastases. Clin Spine Surg 2017;30:E1082-7. [PubMed]
- Huang TJ, Hsu RW, Li Y, et al. Minimal Access Spinal Surgery (MASS) in Treating Thoracic Spine Metastasis. Spine (Phila Pa 1976) 2006;31:1860-3. [Crossref] [PubMed]
- Kumar N, Malhotra R, Maharajan K, et al. Metastatic Spine Tumor Surgery: A Comparative Study of Minimally Invasive Approach Using Percutaneous Pedicle Screws Fixation Versus Open Approach. Clin Spine Surg 2017;30:E1015-21. [PubMed]
- Lau D, Chou D. Posterior thoracic corpectomy with cage reconstruction for metastatic spinal tumors: comparing the mini-open approach to the open approach. J Neurosurg Spine 2015;23:217-27. [Crossref] [PubMed]
- Miscusi M, Polli FM, Forcato S, et al. Comparison of minimally invasive surgery with standard open surgery for vertebral thoracic metastases causing acute myelopathy in patients with short- or mid-term life expectancy: surgical technique and early clinical results. J Neurosurg Spine 2015;22:518-25. [Crossref] [PubMed]
- Stoker GE, Buchowski JM, Kelly MP, et al. Video-assisted thoracoscopic surgery with posterior spinal reconstruction for the resection of upper lobe lung tumors involving the spine. Spine J 2013;13:68-76. [Crossref] [PubMed]
- Fisher CG, DiPaola CP, Ryken TC, et al. A Novel Classification System for Spinal Instability in Neoplastic Disease: An Evidence-Based Approach and Expert Consensus From the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221-9. [Crossref] [PubMed]
- Bilsky MH, Boland PJ, Panageas KS, et al. Intralesional Resection of Primary and Metastatic Sarcoma Involving the Spine: Outcome Analysis of 59 Patients. Neurosurgery 2001;49:1277-86; discussion 1286-7. [Crossref] [PubMed]
- Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine 2010;13:324-8. [Crossref] [PubMed]
- Chi JH, Bydon A, Hsieh P, et al. Epidemiology and Demographics for Primary Vertebral Tumors. Neurosurg Clin N Am 2008;19:1-4. [Crossref] [PubMed]
- Goodwin CR, Khattab MH, Sankey EW, et al. Factors Associated with Life Expectancy in Patients with Metastatic Spine Disease from Adenocarcinoma of the Lung. Global Spine J 2015;5:417-24. [Crossref] [PubMed]
- Kan P, Schmidt MH. Minimally invasive thoracoscopic approach for anterior decompression and stabilization of metastatic spine disease. Neurosurg Focus 2008;25:E8. [Crossref] [PubMed]
- Patil CG, Lad SP, Santarelli J, et al. National inpatient complications and outcomes after surgery for spinal metastasis from 1993–2002. Cancer 2007;110:625-30. [Crossref] [PubMed]
- Ravindra VM, Brock A, Awad A, et al. The role of the mini-open thoracoscopic-assisted approach in the management of metastatic spine disease at the thoracolumbar junction. Neurosurg Focus 2016;41:E16. [Crossref] [PubMed]
- Hirabayashi H, Ebara S, Kinoshita T, et al. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer 2003;97:476-84. [Crossref] [PubMed]
- Goodwin CR, Sankey EW, Liu A, et al. A systematic review of clinical outcomes for patients diagnosed with skin cancer spinal metastases. J Neurosurg Spine 2016;24:837-49. [Crossref] [PubMed]
- Yang SY, Boniello AJ, Poorman CE, et al. A Review of the Diagnosis and Treatment of Atlantoaxial Dislocations. Global Spine J 2014;4:197-210. [Crossref] [PubMed]
- Billinghurst J, Akbarnia BA. Extreme lateral interbody fusion - XLIF. Curr Orthop Pract 2009;20:238-51. [Crossref]
- Shin DA, Kim K, Shin HC, et al. The efficacy of microendoscopic discectomy in reducing iatrogenic muscle injury. J Neurosurg Spine 2008;8:39-43. [Crossref] [PubMed]
- Chaudhary SB, Vives MJ, Basra SK, et al. Postoperative Spinal Wound Infections and Postprocedural Diskitis. J Spinal Cord Med 2007;30:441-51. [Crossref] [PubMed]
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32:470-85. [Crossref] [PubMed]
- Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg 2003;98:149-55. [PubMed]
- Olsen MA, Nepple JJ, Riew KD, et al. Risk Factors for Surgical Site Infection Following Orthopaedic Spinal Operations. J Bone Joint Surg Am 2008;90:62-9. [Crossref] [PubMed]
- O'Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery Clinical article. J Neurosurg Spine 2009;11:471-6. [Crossref] [PubMed]
- Smith WD, Dakwar E, Le TV, et al. Minimally invasive surgery for traumatic spinal pathologies: a mini-open, lateral approach in the thoracic and lumbar spine. Spine (Phila Pa 1976) 2010;35:S338-46. [Crossref] [PubMed]
- McAfee PC, Phillips FM, Andersson G, et al. Minimally invasive spine surgery. Spine (Phila Pa 1976) 2010;35:S271-3. [Crossref] [PubMed]
- Moussazadeh N, Rubin DG, McLaughlin L, et al. Short-segment percutaneous pedicle screw fixation with cement augmentation for tumor-induced spinal instability. Spine J 2015;15:1609-17. [Crossref] [PubMed]
- Regan JJ, Yuan H, McAfee PC. Laparoscopic fusion of the lumbar spine: minimally invasive spine surgery: a prospective multicenter study evaluating open and laparoscopic lumbar fusion. Spine (Phila Pa 1976) 1999;24:402-11. [Crossref] [PubMed]
- Barzilai O, Laufer I, Yamada Y, et al. Integrating Evidence-Based Medicine for Treatment of Spinal Metastases Into a Decision Framework: Neurologic, Oncologic, Mechanicals Stability, and Systemic Disease. J Clin Oncol 2017;35:2419-27. [Crossref] [PubMed]
- Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine 2013;18:207-14. [Crossref] [PubMed]
- Massicotte EM, Foote M, Reddy R, et al. Minimal access spine surgery (MASS) for decompression and stabilization performed as an out-patient procedure for metastatic spinal tumours followed by spine stereotactic body radiotherapy (SBRT): first report of technique and preliminary outcomes. Technol Cancer Res Treat 2012;11:15-25. [Crossref] [PubMed]
- Moulding HD, Elder JB, Lis E, et al. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases Clinical article. J Neurosurg Spine 2010;13:87-93. [Crossref] [PubMed]
- Turel MK, Kerolus M, O'Toole JE. Minimally invasive "separation surgery" plus adjuvsant stereotactic radiotherapy in the management of spinal epidural metastases. J Craniovertebr Junction Spine 2017;8:119-26. [Crossref] [PubMed]


