Percutaneous endoscopic cervical discectomy: a technical review
Introduction
The treatment of cervical disc herniation has evolved since Smith and Robinson (1) used the approach described by Lahey and Warren (2) to access the anterior cervical spine and carry out the first anterior cervical discectomy and fusion (ACDF). Subsequently, Cloward (3) reported the outcomes of cervical interbody fusion using a dowel type graft and introduced the concept of decompression of the neural structures and endplate preparation under direct visualization. These were the bases for what we now know as ACDF. This procedure has demonstrated to be safe, effective, and associated with high fusion rate (4). Because of that is highly used for the treatment of radiculopathy caused by cervical disc herniation (5). However, it is also related to complications such as dysphagia, postoperative hematoma, unilateral recurrent laryngeal nerve (RLN) palsy, cerebrospinal fluid (CSF) leakage, accidental esophageal perforation, worsening of preexisting symptoms (radiculopathy or myelopathy), temporary unilateral Horner syndrome, implant failure, superficial surgical wound infection, adjacent segment disease, pseudoarthrosis and other graft-related problems (6). Technological advances in recent decades have allowed the development of new techniques capable of achieving similar clinical outcomes compared with conventional procedures but with the advantages of shorter hospital stay, lesser tissue damage, reduced blood loss, early functional recovery, among others (7-9). One of these techniques is the well-known percutaneous endoscopic spinal surgery. Initially, the procedure was described by Hijikata (10) and Kambin (11), who independently introduced the concept of percutaneous lumbar nucleotomy, while Tajima et al. (12), reported the first description of the anterior percutaneous endoscopic cervical discectomy (PECD) in 1989. There are two ways to perform PECD: anterior approach and posterior approach which depends on the localization of the herniated disc. The objective of PECD is the decompression of the neural elements (spinal cord or exiting nerve root) under direct visualization through a percutaneous endoscopic approach (13,14). The improvement of the endoscopic instruments has allowed the PECD increases its usefulness. This procedure is no longer a technique used only for intradiscal decompression (automated nucleotomy, chemonucleolysis). Currently, remove central, paracentral or foraminal soft disc herniations by PECD is feasible and devices such as laser or high-speed endoscopic drill allow to do it (15-19). Therefore, PECD could be considered as an alternative to the ACDF, total disc replacement (TDR), and to the posterior microdiscectomy for the treatment of cervical disc herniations. Compared with the procedures mentioned above, PECD offers similar pain relief and advantages such as a clear vision of the surgical target, shorter surgical time, faster recovery, and less damage to tissues (20). In this article reviewed the anterior and posterior PECD.
Anterior PECD
Indications
The success of this procedure depends on the proper selection of patients and adequate decompression of the neural elements (14). Clinical outcomes have shown to be better in patients with lateral soft disc herniations and unilateral radicular pain irradiated to the arm (21). Anterior PECD is effective for central or paracentral disc herniation. Ahn (14) considers the technique as useful for disc herniations medial to the lateral edge of the cervical spinal cord. Patients with radicular syndrome with or without neck pain caused by soft cervical disc herniation diagnosed in magnetic resonance imaging (MRI) and computed tomography (CT). Unsuccessful conservative treatment for 6 weeks, no bony spur larger than 2 mm, regardless of the herniation size, disc space preserved at least 4 mm, and symptoms of the patient concordant with provocative discography. Disc herniations in the segments C4–C5, C5–C6, and C6–C7 are accessible. Approaching the segments C3–C4 or C7–T1 by this technique is uncommon but possible (13,14,20,22).
Contraindications
Several authors have reported the following contraindications: collapsed intervertebral disc space less than 4 mm, calcified or hard disc herniation, and sequestered disc fragment. Instability, cervical spondylotic myelopathy with severe spondylosis or severe neurological deficit, high grade migrated disc herniation, ossification of the posterior longitudinal ligament, history of anterior cervical surgery, and other conditions, such as neoplasms, fracture, infection, and epidural fibrosis (13,14,20,22).
Surgical technique
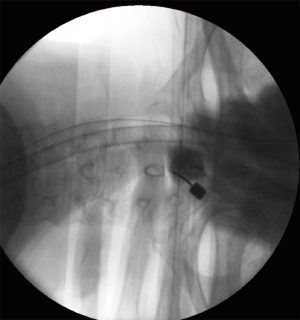
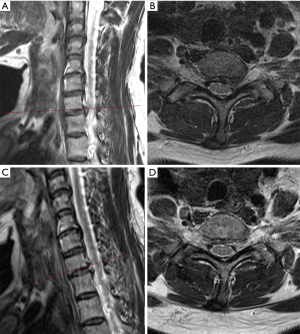
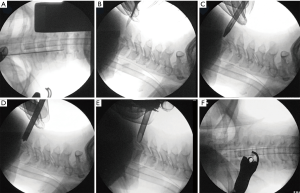
The surgical instruments used for this procedure consists of a 0–25° endoscope with a light source and a working channel for grasping forceps, dissectors, radiofrequency coagulator, and side-firing laser. The surgery is performed with continuous 0.9% saline solution irrigation with the purpose of clear visualization, hemostasis, and neural protection when used radiofrequency or laser. With the patient aware, in a supine position on a radiolucent table and under superficial sedation, the neck is slightly extended. The index level and the midline are marked with the C-arm using an anteroposterior (AP) and lateral views. A local anesthetic is infiltrated in the skin at the entry-point. For paracentral disc herniations, a contralateral approach is preferred, but in case of central disc herniation, a right anterior cervical access is done. The pulse of the carotid artery is felt with the left hand, and the carotid complex is displaced laterally with the middle finger, while the tracheoesophageal complex is mobilized medially with the index finger. Then the tip of the index finger should feel the surface of the vertebral body. After confirming the correct location of the index level using intraoperative fluoroscopy, a spinal needle is inserted gently through the anterior wall of the cervical disc and advanced 5 mm (Figure 1). Subsequently, an intraoperative discography is performed with a mixture of 0.5-mL contrast medium and indigo carmine to differentiate the herniated nucleus tissue during decompression. A guide wire is introduced through the cannulated needle and the annulus. Then, a 3-mm skin incision is made. Afterward, a sequential dilatation with dilators of 1-mm and 2-mm is carried out to place the working cannula as the final step. Then a trephine is inserted through the cannula to cut the annulus. After this, the discectomy can be carried out using microforceps and laser under direct endoscopic visualization. The laser commonly used is the holmium yttrium-aluminum-garnet (Ho:YAG), which ablates and shrink the herniated disc. Enlarge the annular tear for an easy release of the herniated mass it is also possible with this device. Decompression ending when no more disc fragments are visualized directly in the posterior longitudinal ligament through the endoscope or dural sac observed (13,14,22-25). Figure 2 exemplifies the case of a 57-year-old female with radicular pain in the left arm and hyperesthesia in the left C8 dermatome. The patient was diagnosed with a C7–T1 soft cervical disc herniation, and she underwent anterior PECD with favorable postoperative outcomes.
Complications
A low rate of complications in patients underwent anterior PECD has been reported. However, they can be serious, and usually are access-related with an early presentation, such as vascular injury, prevertebral hematoma, swallowing dysfunction, esophageal injury, nerve injuries, wound infection, and cervical spinal cord compression (26). Tzaan (25) reported an incidence of 2% in 107 patients treated by the technique. The author referred a patient with postoperative headache, one case of iatrogenic damage of carotid artery, and a patient with recurrence after 2 years since the first procedure. No complications were reported by Oh et al. (26), nevertheless, five patients had cervical stenosis and collapsed disc height, and 4 presented recurrences during the follow-up. Ruetten et al. (27), in a prospective, randomized, controlled study of 49 patients treated by ACDF and 54 by anterior PECD, reported transient difficulty for swallowing in five cases of ACDF and in 2 of anterior PECD. Revision rate of ACDF cases was 6.1% compared with 7.4% of anterior PECD. Recurrence was the main cause for revision. Lee et al. (28) reported long-term outcomes of 37 patients underwent anterior PECD. They found four patients with recurrence and development of progressive kyphosis treated by ACDF procedure. Proper selection of patients suitable for this procedure is essential to avoid induced postoperative kyphosis and others procedure-related complications. Preservation of the anterior two-thirds of the cervical disc could prevent sagittal balance disorders (Table 1).
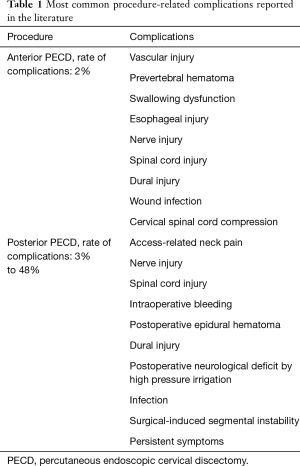
Full table
Technical comments
A precise and direct approach towards the site of pathology, direct visualization of the herniated mass and the preservation of the anatomical planes of the neck are the highlights of anterior PECD. Experience must be gained first performing open anterior cervical approaches and spinal endoscopic procedures. This technique requires a steep learning curve (14). The authors recommend an entry-point as closer as possible to the midline for preventing the injury of longus colli muscles and cervical sympathetic chain (22). A safe zone recommended should be the space between the air column of the airway seen in the AP projection and the carotid pulse (14). Also, strict intraoperative fluoroscopic control during placement of the guide wire, dilators and working cannula inside the disc avoid further catastrophic complications (directly damage of spinal cord). Therefore, the working cannula should be located in the midline on AP projection and should not advance beyond the posterior vertebral line on lateral projection (22). Concerning the success rate, Ahn et al. reported 88.3% of success in 111 subjects (21), Tzaan 91% in 107 patients (25), and 87% in 101 patients by Oh et al. (26). The two major factors associated with excellent long-term outcome are unilateral radiculopathy symptoms and lateral disc herniation (21).
Posterior PECD
The basis of posterior PECD dates since 1944 when Spurling and Scoville proved the effectiveness of a method to treat cervical foraminal stenosis caused by a lateral disc herniation or osteophytes. The approach consisted of an open laminoforaminotomy to decompress the lateral recess and the intervertebral foramen under the direct visualization of the exiting nerve root (29). In appropriately selected patients, posterior laminoforaminotomy results in a success rate of 93% to 97% of patients (30,31). Because an extensive subperiosteal stripping of the cervical paraspinal muscles is avoided with this technique, postoperative neck pain is minimal or non-existent (32). Subsequently, cervical microendoscopic foraminotomy (MEF) was a successful update of the open procedure, demonstrating lesser damage to cervical muscles and similar or better visualization than the previous technique (33,34). Later, Ruetten et al. (35) described the full-endoscopic posterior foraminotomy for cervical disc herniations.
Indications
The following are indications for posterior PECD: foraminal cervical disc herniations located lateral to the edge of spinal cord in MRI and CT scan, from C2–C3 to C7–T1. Unilateral cervical radiculopathy with pain irradiated to the arm. Foraminal stenosis with unilateral symptoms. Lateral craniocaudal sequestered discs. Fail to conservative treatment for at least six weeks (14,35).
Contraindications
The following contraindications have been described in the literature; segmental instability, cervical deformity, central stenosis, a medial location of the herniated disc, extradural lesions mimicking a lateral or foraminal disc herniation (14,35).
Surgical technique
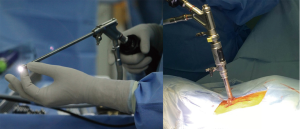
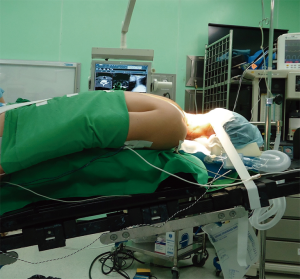
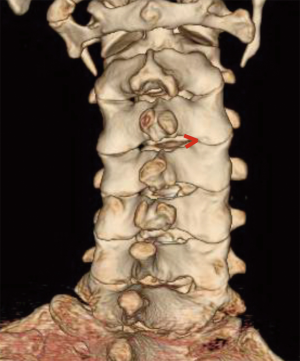
The surgical instruments used in this technique are a little different from those used in anterior PECD. The endoscope and working cannula are larger because the decompression and discectomy are not intradiscal as in the anterior PECD (Figure 3). Endoscopic drill and kerrison punch can be used for performing the foraminotomy or laminoforaminotomy (Figure 4). A 6.9-mm outer diameter endoscope with an angle vision of 25° and a working cannula with an outer diameter of 7.9-mm and a beveled opening are also used. Continuous irrigation of saline solution and radiofrequency probe are also needed. The surgery is performed under general anesthesia with intraoperative fluoroscopy. The patient is placed in prone and the head is fixed with a tape. The surgical table is tilted for a reverse Trendelenburg (Figure 5). Additional head-fixation devices are not necessary. To identify the entry-point the lamino-facet junction should be seen on the AP view at the index level. A 25-cm and 18-gauge needle is advanced and placed on this site. A 9-mm skin incision is made and fascia is cut. Then, the obturator is introduced and used to feel the inferior border of the upper laminae, the superior edge of the inferior laminae, and the medial point of the facet joint. These anatomical landmarks seem like a “V” letter their confluence is called “V” point (Figure 6). Consequently, the beveled working cannula is inserted and obturator removed (Figure 7). At this point, the endoscope can be introduced through the working cannula, and the continuous irrigation system is opened. The overlying soft tissue is coagulated with the RF probe and removed with endoscopic forceps. Once the osseous structures have been exposed, the lower border of the superior laminae is drilled until expose the attachment of the ligamentum flavum, and the drill is directed laterally toward the facet joint and caudally toward the cervical pedicle. Finally, intersection of the ascending facet with the inferior laminae is drilled. Subsequently, the ligamentum flavum and foraminal ligament are removed to expose the exiting nerve root and underlying disc space using a dissector and punches. It is necessary to feel with a nerve hook the medial wall of the pedicle to avoid excessive removal of the facet joint. After proper exposure of the exiting nerve root, the intervertebral disc can be explored. The dissection of herniated disc can be performed through the axilla or shoulder of the exiting nerve root depending on the site of pathology. It is also possible to observe the medial margin of the spinal cord for an adequate orientation and decompression of exiting nerve root. After a successful discectomy, the exiting nerve root should feel free when it is palpated with a nerve hook (14,35,36). Figure 8 shows the case of a 24-year-old male with severe neck and left shoulder pain. The patient was diagnosed with a C3–C4 left foraminal cervical disc herniation. Postoperative good outcomes were noted in the patient.
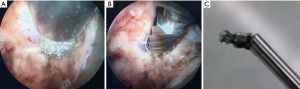
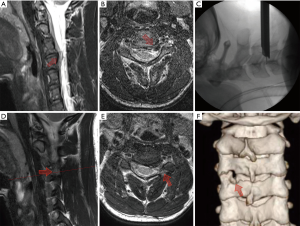
Complications
Complications such as access-related neck pain, injury or irritation of spinal nerves and spinal cord, intraoperative bleeding or postoperative epidural bleeding, dural injury, headache, seizures or postoperative neurological deficit caused by the increased pressure of continuous irrigation system, infections, impaired wound healing, surgical-induced instability, and persistent symptoms, could happen (37,38).
Yang et al. in a retrospective and comparative study of anterior and posterior PECD (42 patients in every group) reported a rate of complications of 7.1% (3/42) in patients underwent anterior PECD and 4.8% (2/42) in patients underwent posterior PECD. The authors observed a transient pain in the contralateral side in one patient by excessive manipulation of the myelon during the procedure and another case for surgical revision after posterior PECD (37). In a prospective study of 28 patients, 8 complained of neck pain. The authors established that highly increased cervical epidural pressure by continuous saline irrigation was the main cause (39). Two cases of approach-related intraoperative total spinal anesthesia during posterior PECD were reported by Wu et al. (40). Perforation on C6 lamina with the spinal needle during the approach lead anesthetics went through the iatrogenic hole to subarachnoid space. Ruetten et al. in 2008 reported an incidence of 3% of complications and a rate of recurrence of 3.4% in 89 patients underwent posterior PECD. All complications reported were dermatomal hyperesthesia in the arms (Table 1) (41).
Technical comments
A concern regarding posterior PECD is that exploration and dissection of the epidural space can cause significant bleeding and difficult the visibility through the endoscope. Therefore, control of the bleeding from cervical venous plexus should be a priority in this technique. The use of RF probe and hemostatic agents can be useful at this step. The beveled working cannula can be used as a protector of the exiting nerve root during the exploration of disc space or the discectomy (14). Facet joint removal should be less than 50% to avoid procedure-induced segmental instability (42), and radiographic follow-up done in patients with less than 10° cervical lordosis because of the higher risk of progressive segmental kyphosis (43). The outcomes reported in a study of 32 patients with foraminal disc herniation and unilateral radiculopathy treated by posterior PECD were good in 91% of patients at the end of follow-up (mean of 30±7 months). Radiological outcomes demonstrated that cervical curvature, segmental angle, anterior, and posterior height were not significantly changed in patients with less and more than 10 degrees of cervical lordosis, concluding that cervical curvature does not worsen after posterior PECD (44). In 100 patients studied by Ruetten et al. (35), medication for access-related pain was not required in all patients after discharge, and after 2 years, 76 patients presented complete relief of their pain, 8 occasional pain or clearly-reduced pain, and 3 no improvement. In another randomized control trial (RCT) of full-endoscopic posterior PECD versus standard ACDF for soft cervical disc herniations published by Ruetten et al. (41), in 175 patients followed for two years, the authors reported 87.4% of good outcomes, concluding that posterior PECD is a safe and effective alternative to conventional surgery in patients properly selected. Among the advantages of this technique, there is no risk of injury for anterior structures of the neck such as trachea, esophagus, carotid artery, thyroid, RLN, and jugular vein. There is a minimal retraction of the cervical muscles, and the technique is highly precise for the pathology. Moreover, visualization under the endoscope with a light source and the continuous irrigation system is very effective. Before starting with the use of the technique, the surgeon should take into account the following. The procedure has a steep learning curve, orientation through the endoscope specially for beginner surgeons could be difficult. The full-endoscopic techniques differ from the microscopic or microendoscopic technique in the way for working with the surgeon’s hands. Endoscopic surgeon works with one hand while the other guide the endoscope. In the other techniques, each hand is used for the surgical instruments (suction and working tools) (35,36).
Conclusions
Endoscopic techniques for cervical discectomy (anterior and posterior) have shown good clinical outcomes with low rate of complications. These procedures performed by experienced endoscopic surgeons in properly selected patients are feasible and effective. Each approach (anterior and posterior) has to be considered based on the location of the pathology and other indications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 1958;40-A:607-24. [Crossref] [PubMed]
- Lahey FH, Warren KW. Esophageal diverticula. Surg Gynecol Obstet 1954;98:1-28. [PubMed]
- Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg 1958;15:602-17. [Crossref] [PubMed]
- Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine 2007;6:298-303. [Crossref] [PubMed]
- Song KJ, Choi BY. Current concepts of anterior cervical discectomy and fusion: a review of literature. Asian Spine J 2014;8:531-9. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. [Crossref] [PubMed]
- Choi G, Pophale CS, Patel B, et al. Endoscopic Spine Surgery. J Korean Neurosurg Soc 2017;60:485-97. [Crossref] [PubMed]
- Thongtrangan I, Le H, Park J, et al. Minimally invasive spinal surgery: a historical perspective. Neurosurg Focus 2004;16:E13. [Crossref] [PubMed]
- Snyder LA, O'Toole J, Eichholz KM, et al. The technological development of minimally invasive spine surgery. Biomed Res Int 2014;2014:293582. [PubMed]
- Hijikata S. Percutaneous nucleotomy. A new concept technique and 12 years' experience. Clin Orthop Relat Res 1989.9-23. [PubMed]
- Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin Orthop Relat Res 1986.37-43. [PubMed]
- Tajima T, Sakamoto H, Yamakawa H. Diskectomy cervicale percutanee. Rev Med Orthop 1989;17:7-10.
- Choi G, Lee SH, de Carvalho MF, et al. Discectomia endoscópica percutânea cervical: 16 anos de experiência e revisão da literatura. Coluna/Columna 2009;8:344-8. [Crossref]
- Ahn Y. Percutaneous endoscopic cervical discectomy using working channel endoscopes. Expert Rev Med Devices 2016;13:601-10. [Crossref] [PubMed]
- Courtheoux F, Theron J. Automated percutaneous nucleotomy in the treatment of cervicobrachial neuralgia due to disc herniation. J Neuroradiol 1992;19:211-6. [PubMed]
- Hoogland T, Scheckenbach C. Low-dose chemonucleolysis combined with percutaneous nucleotomy in herniated cervical disks. J Spinal Disord 1995;8:228-32. [Crossref] [PubMed]
- Bonaldi G, Minonzio G, Belloni G, et al. Percutaneous cervical diskectomy: preliminary experience. Neuroradiology 1994;36:483-6. [Crossref] [PubMed]
- Siebert W. Percutaneous laser discectomy of cervical discs: preliminary clinical results. J Clin Laser Med Surg 1995;13:205-7. [PubMed]
- Hellinger J. Technical aspects of the percutaneous cervical and lumbar laser-disc-decompression and -nucleotomy. Neurol Res 1999;21:99-102. [Crossref] [PubMed]
- Lee SH, Lee JH, Choi WC, et al. Anterior or minimally invasive approaches for the cervical spine. Orthop Clin North Am 2007;38:327-37. [Crossref] [PubMed]
- Ahn Y, Lee SH, Lee SC, et al. Factors predicting excellent outcome of percutaneous cervical discectomy: analysis of 111 consecutive cases. Neuroradiology 2004;46:378-84. [Crossref] [PubMed]
- Choi G, Uniyal P, Hassan Z, et al. A new progression towards a safer anterior percutaneous endoscopic cervical discectomy: A technical report. J Spine 2016;5:329. [Crossref]
- Ahn Y, Lee SH, Shin SW. Percutaneous endoscopic cervical discectomy: clinical outcome and radiographic changes. Photomed Laser Surg 2005;23:362-8. [Crossref] [PubMed]
- Ahn Y, Lee SH, Chung SE, et al. Percutaneous endoscopic cervical discectomy for discogenic cervical headache due to soft disc herniation. Neuroradiology 2005;47:924-30. [Crossref] [PubMed]
- Tzaan WC. Anterior percutaneous endoscopic cervical discectomy for cervical intervertebral disc herniation: outcome, complications, and technique. J Spinal Disord Tech 2011;24:421-31. [Crossref] [PubMed]
- Oh HS, Hwang BW, Park SJ, et al. Percutaneous endoscopic cervical discectomy (PECD): An analysis of outcome, causes of reoperation. World Neurosurg 2017;102:583-92. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int Orthop 2009;33:1677-82. [Crossref] [PubMed]
- Lee JH, Lee SH. Clinical and radiographic changes after percutaneous endoscopic cervical discectomy: a long-term follow-up. Photomed Laser Surg 2014;32:663-8. [Crossref] [PubMed]
- Spurling RS, Scoville WB. Lateral rupture of the cervical intervertebral disc: A common cause of shoulder and arm pain. Surg Gynecol Obstet 1944;78:350-8.
- Henderson CM, Hennessy RG, Shuey HM Jr, et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery 1983;13:504-12. [Crossref] [PubMed]
- Zeidman SM, Ducker TB. Posterior cervical laminoforaminotomy for radiculopathy: review of 172 cases. Neurosurgery 1993;33:356-62. [PubMed]
- O’Toole JE, Sheikh H, Eichholz KM, et al. Endoscopic posterior cervical foraminotomy and discectomy. Neurosurg Clin N Am 2006;17:411-22. [Crossref] [PubMed]
- Burke TG, Caputy A. Microendoscopic posterior cervical foraminotomy: a cadaveric model and clinical application for cervical radiculopathy. J Neurosurg 2000;93:126-9. [PubMed]
- Roh SW, Kim DH, Cardoso AC, et al. Endoscopic foraminotomy using MED system in cadaveric specimens. Spine (Phila Pa 1976) 2000;25:260-4. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: prospective 2-year results of 87 patients. Minim Invasive Neurosurg 2007;50:219-26. [Crossref] [PubMed]
- Wagner R, Telfeian AE, Iprenburg M, et al. Minimally invasive fully endoscopic two-level posterior cervical foraminotomy: technical note. J Spine Surg 2017;3:238-42. [Crossref] [PubMed]
- Yang JS, Chu L, Chen L, et al. Anterior or posterior approach of full-endoscopic cervical discectomy for cervical intervertebral disc herniation? A comparative cohort study. Spine (Phila Pa 1976) 2014;39:1743-50. [Crossref] [PubMed]
- Komp M, Oezdemir S, Hahn P, et al. Full-endoscopic posterior foraminotomy surgery for cervical disc herniations. Oper Orthop Traumatol 2018;30:13-24. [Crossref] [PubMed]
- Joh JY, Choi G, Kong BJ, et al. Comparative study of neck pain in relation to increase of cervical epidural pressure during percutaneous endoscopic lumbar discectomy. Spine (Phila Pa 1976) 2009;34:2033-8. [Crossref] [PubMed]
- Wu W, Yan Z. Intraoperative total spinal anesthesia as a complication of posterior percutaneous endoscopic cervical discectomy. Eur Spine J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:940-8. [Crossref] [PubMed]
- Adamson TE. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg 2001;95:51-7. [PubMed]
- Jagannathan J, Sherman JH, Szabo T, et al. The posterior cervical foraminotomy in the treatment of cervical disc/osteophyte disease: A single-surgeon experience with a minimum of 5 years´ clinical and radiographic follow-up. J Neurosurg Spine 2009;10:347-56. [Crossref] [PubMed]
- Kim CH, Shin KH, Chung CK, et al. Changes in cervical sagittal alignment after single-level posterior percutaneous endoscopic cervical diskectomy. Global Spine J 2015;5:31-8. [Crossref] [PubMed]


