Osimertinib in first-line treatment—is a comparison not proof?
Considerable progress has been made in the treatment of advanced non-small-cell lung carcinoma (NSCLC) harboring an epidermal growth factor receptor (EGFR) gene mutation (EGFR-NSCLC) since the publication of the BR21 trial (1). That trial showed erlotinib to have a modest effect compared with placebo in the second and subsequent lines of treatment in the all-comers NSCLC population. Because the effect was most pronounced in Asians and non-smokers, it was soon revealed that responders harbored an activating mutation of EGFR (2). Further advances have since been made by breaking down the various types of mutations into common, rare, and complex (3), by developing molecular techniques to screen real-world patients for these mutations (4), and by conducting randomized controlled therapeutic trials that compared the efficacy and safety of tyrosine kinase inhibitors of EGFR (EGFR-TKIs) against successive standards of care (summarized in Tables 1 and 2). However, as the French proverb has it, comparaison n’est pas raison (a comparison is not proof).

Full table

Full table
Comparison between tyrosine kinase inhibitors and chemotherapy (CT) proves conclusive in the 1st line
At least eight phase III therapeutic clinical trials (Table 1) (5-12) have shown that first- (erlotinib, gefitinib) and second-generation (afatinib) EGFR-TKIs were safer and more effective and resulted in better quality of life than a doublet regimen of platinum-based CT. The trials also showed that: (I) only L858R mutations and exon 19 deletions (common mutations) clearly benefited from TKIs; (II) tumor progression occurred between a median of 9 and 12 months after TKI initiation; (III) the brain was the most common site of recurrence; (IV) molecular mechanisms could be identified by performing a rebiopsy of the tumor or analyzing circulating tumor DNA (ctDNA) (2,17). But the trials did not show whether it was worth beginning the treatment sequence with a TKI rather than with CT, nor did it show us which TKI was most effective. The LUX-lung 3 and 6 trials’ planned subgroup analysis by mutation (L858R vs. del19) (11,12) and pooled analysis (18) finally revealed that to begin treatment by a TKI rather than by a CT resulted in better overall survival (OS), particularly in the del19 subgroup suggesting the importance of the therapeutic sequence.
Comparison between 1st and 2nd generations proves inconclusive in the 1st line
Two trials (Table 2) then compared the efficacy of first- (gefitinib) and second-generation (afatinib, dacomitinib) TKIs. The LUX-lung 7 trial (13,14) statistically demonstrated afatinib to have better efficacy than gefitinib based on a reduced risk of progression on afatinib. However, the curves did not separate until after 12 months, OS was the same and the proportion of patients who had grade 3 or more adverse events was twice as high on afatinib. The more recent ARCHER 1050 trial (15) clearly showed the superiority of dacomitinib over gefitinib in first-line treatment as the PFS curves separated after 6 months of treatment, although that trial only included Asian patients and did not include any patients with brain metastasis. What is more, 64% of patients suffered adverse events of grade ≥3 on dacomitinib and the median survival data was not mature. The role of second-generation TKIs in the first line is hard to clearly define, and they have no proven utility in the second line after first-generation TKIs fail.
Comparison between osimertinib and CT proves conclusive in the 2nd line for T790M-EGFR-NSCLC
Osimertinib is a third-generation irreversible TKI specifically for EGFR-mutated forms that is active in vitro and in phase I and II trials against T790M resistance mutations (19,20). It is also characterized by very good brain exposure, displaying a cerebrospinal fluid to plasma concentration ratio of 0.39 (17). With the results of the AURA 3 trial (21), osimertinib soon proved its value in the second line compared with a doublet regimen of platinum-based CT plus pemetrexed in patients who progressed while on a first or second-generation TKI. Eligible patients were those whose tumor harbored T790M mutation identified by rebiopsy or ctDNA analysis. Median PFS was higher in the osimertinib arm (10.1 vs. 4.4 months) with the risk of progression or death reduced by 70% [hazard ratio (HR): 0.30; 95% confidence interval (CI), 0.23 to 0.41; P<0.001]. A similar effect was observed in all patient subgroups, and particularly in patients with cerebral metastases. The proportion of grade ≥3 adverse events was lower in the osimertinib arm, and quality of life also favored the TKI arm. OS data has yet to be reported however. Lastly, new resistance mechanisms (22-24) have been identified and include: (I) new acquired resistance mutations (such as C797S) of the EGFR gene in addition to the T790M mutation; (II) loss of the T790M mutation; (III) acquisition of mutations in the intracellular signaling pathways (such as RAS/RAF, MEK, PI3K, JAK); (IV) amplification of a parallel signaling pathway (MET, HER2, FGFR) that leads to bypass of the EGFR pathway; and (V) histological transdifferentiation, particularly into small-cell lung carcinoma.
Comparison between osimertinib and 1st/2nd TKIs favors osimertinib in the 1st line
Even more recently, osimertinib has been propelled into first-line treatment, initially following the results of a phase I trial (25) but especially during the European Society of Oncology Congress with the communication of results from the FLAURA trial (16) investigating the efficacy of osimertinib in the first line compared with first-generation TKIs (gefitinib, 64%; erlotinib, 36%) in NSCLC patients with common EGFR mutations (del19, 63%; L858R, 37%). Median PFS was higher in the osimertinib arm (18.9 vs. 10.2 months) with the risk of progression or death reduced by 54% (HR: 0.46; 95% CI, 0.37 to 0.57; P<0.0001), while the survival curves separated within the first weeks of treatment. A similar effect was observed in all patient subgroups, especially in non-Asians (HR =0.34), male subjects (HR =0.58), smokers (HR =0.48), patients with cerebral metastases (HR =0.47), and patients with L858R mutations (HR =0.51). Even though the response rate did not differ between the two treatment arms, the duration of treatment was practically double in the osimertinib arm (17.2 vs. 8.5 months). The proportion of treatment-related grade ≥3 adverse events was lower in the osimertinib arm (18% vs. 28%). Notably there were clearly fewer cases of hepatic (25% vs. 48%) and cutaneous (9% vs. 25%) toxicity of all grades. Data on quality of life was, however, not reported. Finally, interim analysis—although only 25% mature—showed promising survival with a 47% reduction in the risk of death in favor of osimertinib versus the first-generation TKIs [HR: 0.63; 95% CI, 0.45 to 0.88; P=0.0068 (not significant), while P<0.0015 expected]. Nevertheless, we do not currently have the data to define the type of clinical progression (slow vs. rapid, cerebral vs. systemic) and resistance mechanisms that will be induced by using osimertinib in first-line treatment (25).
Comparison proves inconclusive for choosing the best treatment sequence
The best treatment sequence for any patient being followed for advanced EGFR-mutated NSCLC is the one that provides the longest OS with an acceptable safety profile and maintained quality of life. Hence OS for real-world patients is determined by summing the treatment durations for each line of treatment administered, be it TKI, CT, or local treatment. This sum cannot be simply anticipated by the sum of median PFS figures as observed in the different therapeutic trials. This is because the populations are highly selective, the effectiveness of previous treatments is unknown, post-progression treatment options are not considered, and the biological impact of each line on the next is also unknown. Only strategy trials will be able to elucidate this question.
Nevertheless, Figure 1 summarizes the main PFS and OS data from the various phase III trials while factoring in international guidelines on the management of NSCLC. Sequence 1 is obviously taken from the phase III trials of first- and second-generation TKIs evaluated in the first line of treatment. In those trials, between 44% and 65% of patients were able to receive a second line of CT in the end (8-12), while the IMPRESS trial showed that CT (pemetrexed/platinum doublet and pemetrexed maintenance) resulted in a median PFS of 5 months in second-line treatment (26). With this treatment sequence, OS varies considerably between 19 and 36 months. These differences in OS depend not only on access to medication for subsequent treatment lines but also on differing medical practices in real-world patients, including pursuing TKI beyond progression which prolongs median PFS by 3 months in around 45% of patients with or without locoregional therapy in cases of oligo progression (27). Sequence 2 will certainly soon predominate in countries where it is possible to screen for T790M mutation using rebiopsy and/or ctDNA and where osimertinib will be available, but this will apply to less than 50% of patients who progress—in other words, not to patients who die during first-line treatment, not to patients whose T790M mutation cannot be established, and not to patients who are indeed T790M-negative—with the others continuing to receive CT.
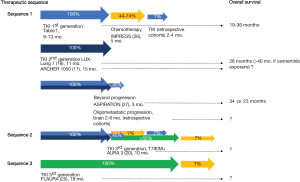
The results of the FLAURA trial may bear out the comparison, especially since this sequence ensures that 100% of patients are exposed to osimertinib, but the evidence is still inconclusive for adopting sequence 3, in particular because of the lack of OS data. In the future, it seems vital to consider strategy trials or at least to obtain data from large real-world cohorts to improve our knowledge of the prognostic impact of treatment sequences. The utility of first-line combination strategies based on first- and second-generation TKIs must continue to be assessed (with anti-angiogenesis, immunotherapy, anti-HER, anti-MET) (17,28). Moving osimertinib to the first line of treatment will cancel out the utility of analyzing ctDNA upon progression, restricted to the emergence of a single resistance mechanism, and it will make it necessary to evaluate next-generation sequencing against rebiopsy results. Finally, this new data will again raise the question of the role of first-line combinations with osimertinib to prevent the emergence of resistance mechanisms independent of the EGFR pathway, which may come to predominate. In any event, CT will continue to play a significant role in treatment sequences for managing EGFR-mutated NSCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author declares conflicts of interest with Astra-Zeneca, Roche, Bohringer Ingelheim receiving personal fees for participating to expert board or being an investigator of several trials.
References
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Cadranel J, Ruppert AM, Beau-Faller M, et al. Therapeutic strategy for advanced EGFR mutant non-small-cell lung carcinoma. Crit Rev Oncol Hematol 2013;88:477-93. [Crossref] [PubMed]
- Leduc C, Merlio JP, Besse B, et al. Clinical and molecular characteristics of non-small-cell lung cancer (NSCLC) harboring EGFR mutation: results of the nationwide French Cooperative Thoracic Intergroup (IFCT) program. Ann Oncol 2017;28:2715-24. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkist J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 2017;17:637-58. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: aura study phase ii extension component. J Clin Oncol 2017;35:1288-96. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Eberlein CA, Stetson D, Markovets AA, et al. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on ras signaling in preclinical models. Cancer Res 2015;75:2489-500. [Crossref] [PubMed]
- Ercan D, Choi HG, Yun CH, et al. EGFR mutations and resistance to irreversible pyrimidine-based egfr inhibitors. Clin Cancer Res 2015;21:3913-23. [Crossref] [PubMed]
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib as first-line treatment of EGFR Mutation-positive advanced non-small-cell lung cancer. J Clin Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015;16:990-8. [Crossref] [PubMed]
- Park K, Yu CJ, Kim SW, et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION study. JAMA Oncol 2016;2:305-12. [Crossref] [PubMed]
- Cortot AB, Moro-Sibilot D, Cadranel J. Afatinib + cetuximab first-line in egfr-mutant lung cancer--letter. Clin Cancer Res 2016;22:1827. [Crossref] [PubMed]


