On predicting clinical response to chemoradiotherapy in esophageal squamous cell carcinoma: additional evaluation by magnetic resonance imaging may help
The current standard treatment options for locally advanced esophageal squamous cell carcinoma (ESCC) include neoadjuvant chemoradiotherapy (CRT) followed by esophagectomy and definitive CRT (1-4). For patients who receive neoadjuvant CRT, pathologic tumor response, including pathologic complete response (pCR) and tumor regression grade, is predictive of patients’ survival (5,6). For patients who receive definitive CRT, clinical complete tumor response has also been shown to associate with patients’ survival (7). However, the clinical tumor response evaluation for locally advanced esophageal cancer is limited by inferior sensitivity and specificity of conventional imaging modalities (8). For example, although endoscopy can evaluate the response of intraluminal esophageal tumor and can confirm the suspicious lesions by biopsy, it could not detect residual tumor deep in muscular layer and adventitia of esophageal wall. The application of computed tomography (CT) to evaluate esophageal tumor response is frequently hampered by consequences of CRT, such as edema, fibrosis, and necrosis. A better clinical tumor response evaluation is warranted to help prognosis prediction in patients of locally advanced ESCC receiving definitive CRT.
High resolution T2-weighted magnetic resonance imaging (MRI) has been studied for staging of esophageal cancer (9,10). Compared with CT, T2-weighted MRI can show clear layers of esophageal wall, the surrounding structure, and the depth of tumor invasion. Diffusion-weighted MRI (DWI), a functional imaging, has also been investigated in esophageal cancer (11). Apparent diffusion coefficient (ADC), a parameter calculated from DWI, represents the level of free diffusion of water. A retrospective study of ESCC patients demonstrated that lower ADC measured in their tumors, meaning restricted diffusion of water in higher cellularity, was associated with worse prognosis (12). ADC has also been studied in evaluating tumor response to CRT in several cancer types, including esophageal cancer (13,14). However, MRI is less sensitive than endoscopy in detecting intraluminal esophageal tumors and less sensitive than CT in detecting small lung metastases. Therefore, it has been hypothesized that adding MRI studies to the conventional CT and endoscopy would improve the predictive accuracy of the clinical tumor response evaluation in ESCC patients treated with CRT.
Qiu and colleagues recently published a clinical study evaluating the prognosis prediction performance of combining MRI, endoscopy and CT in patients with loco-regional ESCC treated with definitive CRT (15). The study enrolled 67 patients with clinical stages II to IVb ESCC according to American Joint Committee on Cancer (AJCC) 6th edition, and compared the traditional criteria using CT and endoscopy with the new criteria using additional T2-weighted and diffusion-weighted MRI studies in evaluating the clinical response to definitive CRT. The complete response defined by the traditional criteria comprised disappearance of primary tumor at endoscopy and CT (esophageal wall thickness <10 mm) and pathologic lymph nodes at CT (short axis <10 mm); and the complete response defined by the new criteria comprised no intraluminal tumor at endoscopy, no new metastasis at CT, normalization or subtle hypo-dense thickness of esophageal wall and no involved lymph nodes at T2-weighted MRI, and ADCpost of primary tumor ≥2.64×10‒3 mm2/s. Among 67 enrolled patients, 10 (14.9%) were classified as complete responders by the traditional criteria, whereas 37 (55.2%) were classified as complete responders by the new criteria incorporating MRI studies. Using 12-month progression-free survival (PFS) as a surrogate of treatment efficacy, the sensitivity was much higher for the new criteria than the traditional criteria (82.4% vs. 20.6%) and the specificity for the two criteria was very similar (88.9% vs. 92.6%). Complete response defined by the new criteria, rather than that by the traditional criteria, was an independent prognostic factor for PFS (hazard ratio: 0.114, P<0.0001). The authors concluded that tumor responses evaluated by the new criteria combining MRI, endoscopy and CT is highly predictive of the prognosis for ESCC patients treated with definitive CRT.
Qiu and colleagues are congratulated for publishing the data supporting the superiority of combining MRI studies with conventional endoscopy and CT as the evaluation of clinical tumor response to CRT in ESCC patients. However, caution needs be exercised when interpreting their results. The report was based on a single-institute cohort study and its analysis was likely performed retrospectively. The finding of improved prediction of PFS by the new criteria was not validated by independent patient cohorts. According to the authors, histologically diagnosed ESCC patients with stage I–IVb disease who underwent definitive CRT were enrolled between 2011 and 2015. However, it was not clearly mentioned in the report what criteria were used to select patients for definitive CRT, but not other treatment options such as neoadjuvant CRT or radical surgery. The way patients were selected may lead to certain bias. Patients enrolled received two different chemotherapy regimens, nedaplatin plus S-1 and nedaplatin plus docetaxel, both of which are not commonly used in most parts of the world. Further, it is not clear why the authors used AJCC TNM system 6th version to stage their patients enrolled between 2011 and 2015 since the AJCC TNM 7th edition was launched in 2009. Lastly, the authors used 12-month PFS as a surrogate of treatment efficacy and for the primary analysis in this study. Although this may seem to be reasonable because the median follow-up period for the entire cohort was relatively short (only 15.3 months), survival endpoints with longer periods are preferred to truly reflect the efficacy of definitive CRT and the prognosis of loco-regional ESCC.
Despite the aforementioned limitations, the study does provide important information that may have significant clinical implications. The addition of MRI to the clinical tumor response evaluation could help detect patients with good prognosis who might be missed by the traditional criteria due to the low ability of CT to differentiate post-CRT wall thickness from the presence of residual cancer cells. In the new criteria, MRI evaluates not only wall thickness of the primary tumor site, but also the normalization of esophageal wall layers (by T2-weighted imaging) and the tumor cellularity (by diffusion-weighted imaging). Further studies using a larger patient population and a longer period of follow-up are warranted to validate the findings of this report.
The results of the study may be further investigated to improve our care of patients with locally advanced ESCC. First, according to this study, patients who were categorized as non-complete responders according to the new criteria incorporating MRI studies had a median PFS of 7.8 months and a 2-year PFS of 16%, which were significantly worse than those of complete responders. These non-responders are a potential group of patients indicated for adjuvant or additional therapy to improve their outcome (Figure 1). Second, other imaging modalities such as 18F-fluorodeoxyglucose positron emission tomography (PET) and endoscopic ultrasonography (EUS), that have been widely used for staging ESCC, were not included in the study by Qiu et al. (10). Some studies revealed that the timing of response evaluation by PET may influence the accuracy of survival prediction (16,17). Whether adding PET and EUS to the response evaluation will further improve the predictive accuracy of the new criteria will be an interesting topic of future studies. Third, it is currently unknown whether the new criteria incorporating MRI studies would be helpful in predicting pCR and patients’ outcome in ESCC patients receiving neoadjuvant CRT. Predictive biomarkers of pCR could potentially help stratify patients with different risks for different treatment strategies (Figure 1). For example, those with high probability of pCR after neoadjuvant CRT may consider avoiding esophagectomy and continuing additional CRT as a definitive treatment. Finally, studies have been undergoing to explore molecular and genetic markers, in tumor tissues or in peripheral blood, in order to establish potentially predictive or prognostic biomarkers in ESCC patients treated with CRT.
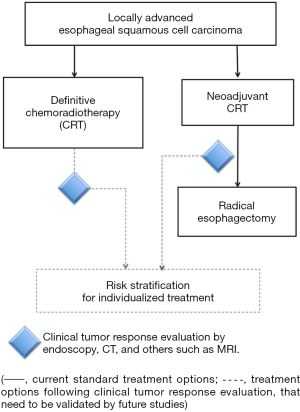
Overall, continuing efforts to refine the clinical tumor response criteria are important to implement risk- stratification and personalized therapy for patients with locally advanced ESCC.
Acknowledgements
Funding: This work was supported by a grant (MOST 105-2314-B-002-186-MY3) from the Ministry of Science and Technology of the Executive Yuan, Taiwan, and a grant NTUH 104-M2891 from National Taiwan University Hospital, Taiwan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- Huang TC, Hsu CH, Lin CC, et al. Systematic review and network meta-analysis: neoadjuvant chemoradiotherapy for locoregional esophageal cancer. Jpn J Clin Oncol 2015;45:1023-8. [Crossref] [PubMed]
- Guo JC, Huang TC, Lin CC, et al. Postchemoradiotherapy pathologic stage classified by the American Joint Committee on the cancer staging system predicts prognosis of patients with locally advanced esophageal squamous cell carcinoma. J Thorac Oncol 2015;10:1481-9. [Crossref] [PubMed]
- Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. [Crossref] [PubMed]
- Bao Y, Liu S, Zhou Q, et al. Three-dimensional conformal radiotherapy with concurrent chemotherapy for postoperative recurrence of esophageal squamous cell carcinoma: clinical efficacy and failure pattern. Radiat Oncol 2013;8:241. [Crossref] [PubMed]
- Cheedella NK, Suzuki A, Xiao L, et al. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: analysis in a large cohort. Ann Oncol 2013;24:1262-6. [Crossref] [PubMed]
- Weijs TJ, Goense L, van Rossum PS, et al. The peri-esophageal connective tissue layers and related compartments: visualization by histology and magnetic resonance imaging. J Anat 2017;230:262-71. [Crossref] [PubMed]
- Qu J, Zhang H, Wang Z, et al. Comparison between free-breathing radial VIBE on 3-T MRI and endoscopic ultrasound for preoperative T staging of resectable oesophageal cancer, with histopathological correlation. Eur Radiol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Aoyagi T, Shuto K, Okazumi S, et al. Evaluation of the clinical staging of esophageal cancer by using diffusion-weighted imaging. Exp Ther Med 2010;1:847-51. [Crossref]
- Liu S, Zhen F, Sun N, et al. Apparent diffusion coefficient values detected by diffusion-weighted imaging in the prognosis of patients with locally advanced esophageal squamous cell carcinoma receiving chemoradiation. Onco Targets Ther 2016;9:5791-6. [Crossref] [PubMed]
- van Rossum PS, van Lier AL, van Vulpen M, et al. Diffusion-weighted magnetic resonance imaging for the prediction of pathologic response to neoadjuvant chemoradiotherapy in esophageal cancer. Radiother Oncol 2015;115:163-70. [Crossref] [PubMed]
- Wang L, Liu L, Han C, et al. The diffusion-weighted magnetic resonance imaging (DWI) predicts the early response of esophageal squamous cell carcinoma to concurrent chemoradiotherapy. Radiother Oncol 2016;121:246-51. [Crossref] [PubMed]
- Qiu B, Wang D, Yang H, et al. Combined modalities of magnetic resonance imaging, endoscopy and computed tomography in the evaluation of tumor responses to definitive chemoradiotherapy in esophageal squamous cell carcinoma. Radiother Oncol 2016;121:239-45. [Crossref] [PubMed]
- Piessen G, Petyt G, Duhamel A, et al. Ineffectiveness of (1)(8)F-fluorodeoxyglucose positron emission tomography in the evaluation of tumor response after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg 2013;258:66-76. [Crossref] [PubMed]
- Chen YM, Pan XF, Tong LJ, et al. Can (1)(8)F-fluorodeoxyglucose positron emission tomography predict responses to neoadjuvant therapy in oesophageal cancer patients? A meta-analysis. Nucl Med Commun 2011;32:1005-10. [Crossref] [PubMed]


