Comparative effectiveness and safety of empagliflozin on cardiovascular mortality and morbidity in adults with type 2 diabetes
Introduction
One of the main goals in managing type 2 diabetes in adults is prevention of cardiovascular morbidity and mortality (1,2). Only a few of the available diabetes medications have shown benefits in reducing cardiovascular risks; most available drug classes such as thiazolidinediones, sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-glucose cotransporter 2 (SGLT2) inhibitors have been approved based on their ability to decrease glycosylated hemoglobin A1c (HbA1c) rather than their ability to prevent morbidity and mortality (3,4). Network meta-analyses of SGLT2 inhibitors suggest lower risk of all-cause and cardiovascular mortality from 3 oral SGLT2 inhibitors combined, at the expense of higher risk of nonfatal stroke [pooled relative risk (RR) 1.30; 95% confidence interval (CI): 1–1.68], genital infection (pooled RR 4.75; 95% CI: 4.00–5.63), and volume depletion (pooled RR 1.53; 95% CI: 1.27–1.83) (5,6). However, the reduction in the risk of mortality and morbidity is mostly attributable to one drug, empagliflozin, the only drug approved by the FDA in 2016 to reduce the risk of cardiovascular death in adult patients with type 2 diabetes mellitus and established cardiovascular disease (5,6).
Older meta-analyses focused on intermediate outcomes of empagliflozin when compared with placebo; e.g., HbA1c, blood pressure, and body weight (7-15). The most recent high-quality meta-analyses included 13 (6) and 16 (5) randomized controlled trials (RCTs) that reported mortality and morbidity in adults with type 2 diabetes treated with empagliflozin but did not examine the comparative effectiveness of empagliflozin and other specific antidiabetic drugs (5,6). Clinicians have to select specific drugs for individual patients rather than relying on drug class benefits and harms.
To support clinical decisions at point of care with all available evidence, we conducted a rapid review of the published and unpublished data from the recently completed RCTs, meta-analyses of RCTs, and primary observational studies that compared the effects of empagliflozin with those of other antidiabetic drugs on all-cause and cardiovascular mortality and morbidity.
Methods
We used a standard recommended methodology in conducting systematic literature reviews and meta-analyses from the Cochrane Collaboration and the Agency for Healthcare Research and Quality (16,17). We developed a priori protocol for a systematic literature review to answer the clinical question about the efficacy and comparative effectiveness of empagliflozin and other antidiabetic medications against mortality and cardiovascular morbidity in adults with type 2 diabetes.
We defined the target population as adults with type 2 diabetes. Eligible interventions included SGLT2 inhibitor empagliflozin when compared with placebo or other antidiabetic medications. Eligible outcomes included all-cause and underlying cause-specific mortality, myocardial infarction (MI), stroke, incidence or progression of heart failure, and hospitalizations for major cardiovascular events. Intermediate outcomes included diabetes control as HbA1c <7% or as defined in the primary studies. We reviewed the frequency and severity of hypoglycemia as well as any harms from examined treatments.
We conducted a comprehensive search in PubMed, EMBASE, the Cochrane Library, www.clinicaltrials.gov and PharmaPendium (www.pharmapendium.com) up to May 2017 to find systematic reviews, published and unpublished RCTs, and nationally representative controlled observational studies that reported adjusted effect estimates (16,17). All of the authors determined the studies’ eligibility. All citations found during the searches are stored in a reference database.
The data was extracted from the Clinical Trials Transformation Initiative (CTTI) (https://www.ctti-clinicaltrials.org/aact-database), checked for quality, and stored in the HPCC platform (High-Performance Computing Cluster, https://hpccsystems.com/).
We performed direct frequentist meta-analyses of aggregate data when definitions of the active and control intervention and patient outcomes were deemed similar for pooling (18). We used random effects models to address inevitable differences in patient characteristics across primary RCTs. For each abstracted hypothesis, we calculated absolute risk difference and RR with 95% CI. We calculated number needed to treat (NNT) and number of attributable events per 1,000 treated with 95% CI based on statistically significant differences in absolute risks of the outcomes. We examined consistency in results across studies with chi-square tests and I2 statistics and concluded statistically significant heterogeneity if I2 was >50% (16). Statistically significant heterogeneity did not preclude statistical pooling (18). However, we planned exploring heterogeneity with a priori defined patient characteristics, drug doses, and study quality if this information was available in the studies (18).
We used consensus method guidelines for systematic review and meta-analyses that do not recommend conducting post hoc analyses of statistical power (19-22). Instead, we downgraded our confidence in true treatment effects based on calculated optimal information size as the number of patients required for an adequately powered individual trial (23). Since power is more closely related to number of events than to sample size, we concluded imprecision in treatment effects if fewer than 250 patients experienced the event (23).
We used Statistics/Data Analysis, STATA software (StataCorp LP, College Station, Texas). Statistical significance was evaluated at a 95% confidence level.
We evaluated the quality of systematic reviews using the Assessment of Multiple Systematic Reviews (AMSTAR) (24). For primary RCTs, we used the Cochrane risk of bias tool on a 3-point scale: high bias, low bias, and unclear (25,26). A low risk of bias was assumed when RCTs met all the risk-of-bias criteria, a medium risk of bias if at least 1 of the risk-of-bias criteria was not met, and a high risk of bias if 2 or more risk-of-bias criteria were not met. An unknown risk of bias was assigned for the studies with poorly reported risk-of-bias criteria. We assigned high risk of bias to all observational studies.
The authors assigned the quality of evidence ratings as high, moderate, low, or very low, according to risk of bias in the body of evidence, directness of comparisons, precision and consistency in treatment effects, and the evidence of reporting bias, using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (27).
A high quality of evidence was assigned to well-designed RCTs with consistent findings. The quality of evidence was downgraded to moderate if at least 1 of 4 quality of evidence criteria was not met; for example, moderate quality of evidence was assigned if there was a high risk of bias in the body of evidence or if the results were not consistent or precise. The quality of evidence was downgraded to low if 2 or more criteria were not met. We concluded a high risk of bias in the body of evidence if at least one RCT had high risk of bias. We downgraded the quality of evidence when we suspected high risk of publication bias due to unavailability of the results in clinicaltrials.gov or journal articles.
A low quality of evidence was assigned to nonrandomized studies, but the rating was upgraded if there was a strong or dose-response association (28). Evidence was defined as insufficient when no studies provided valid information about treatment effects. This approach was applied regardless of whether the results were statistically significant.
Results
Our comprehensive search in PubMed, EMBASE, the Cochrane Library, and clinicaltrials.gov up to May 2017 identified 11 meta-analyses, multiple publications as well as unpublished data from 29 RCTs, and one non-randomized study that examined the benefits and harms of empagliflozin in people with type 2 diabetes (5-14,29). We also identified 2 high-quality meta-analyses and multiple publications as well as unpublished data from 9 RCTs that directly compared empagliflozin with other antidiabetic drugs in people with type 2 diabetes (5-7,30-48).
Primary studies enrolled adults with type 2 diabetes and various baseline degrees of cardiovascular risk, permitted administration of metformin and other antidiabetic drugs, and aimed mostly at diabetes control and drug safety. Only one large non-inferiority trial, the EMPA-REG OUTCOME trial, was designed to examine difference in a composite outcome defined as the first occurrence of cardiovascular death, nonfatal MI, or nonfatal stroke in adults with type 2 diabetes and high cardiovascular risk (49).
Efficacy
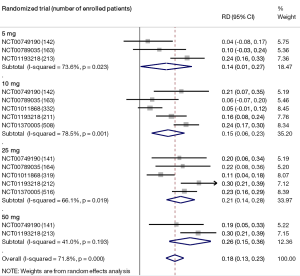
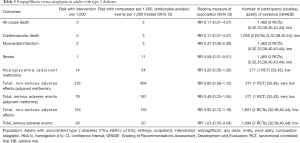
Moderate-quality evidence suggests that empagliflozin reduces all-cause mortality (7,11,40,44,46,47,49-61) and increases the rates of diabetes control without increasing the risk of serious adverse effects and hypoglycemia when compared with placebo in adults with type 2 diabetes (Table 1) (50,51,70). The increase in rates of glycemic improvement starts at the dose of 10 mg/day (150 attributable events per 1,000 treated, Figure 1) and increases to 210 attributable events per 1,000 treated after the larger dose of empagliflozin (25 mg/day, Figure 1).
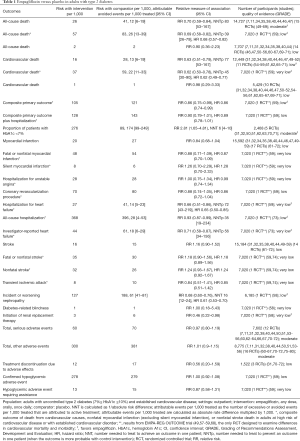
Full table
Low-quality evidence suggests that empagliflozin reduces cardiovascular mortality, the risk of hospitalization for any cause (73), and hospitalizations for heart failure (59), as well as the risk of developing heart failure (73), developing or worsening of nephropathy (58), and the risk of treatment discontinuation due to lack of efficacy, at the expense of higher risk of adverse effects (Table 1) (7,11,40,44,46,47,49-61,70,75-77). The observed improvement in patient outcomes is attributable to the largest RCT, EMPA-REG OUTCOME, which enrolled patients with established cardiovascular diseases (59). Sensitivity analyses excluding this RCT demonstrate no protective effects from empagliflozin against all-cause and cardiovascular mortality in all other RCTs combined (Table 1). There are no differences in the risk of stroke or coronary events between empagliflozin and placebo (Table 1) (40,44,49-59,61,70).
Subgroup analysis of the EMPA-REG OUTCOME trial demonstrates that empagliflozin is better than placebo in reducing the risk of major cardiovascular events in older patients and adults with HbA1c 7.0–8.5% (P value for interaction <0.05, Table S1). Baseline heart failure does not modify empagliflozin effects on cardiovascular mortality and the risk of major cardiovascular effects (data not shown) (73). Empagliflozin is not better than placebo in adults with BMI ≥30 kg/m2 (P value for interaction 0.06, Table S1).
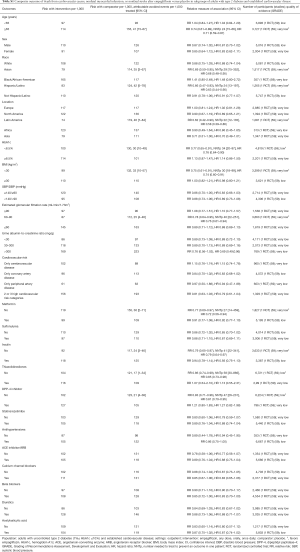
Full table
Safety analyses demonstrate that empagliflozin increases the risk of genital infection, thirst, and polyuria and reduces the risk of acute renal injury and failure, hypertension, and worsening of heart failure (Table S2) (7,11,31,40,50,51,53-56,58,59,62-67,77,78,81-83). Empagliflozin’s safety profile is similar in patients with normal and impaired baseline renal function (Table S2).
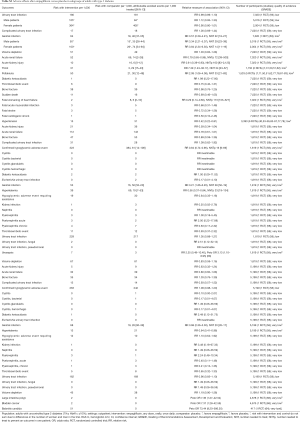
Full table
Post-marketing surveillance suggests more than 1900 case reports of various adverse effects, including fungal and urinary tract infections, diabetic ketoacidosis, unintentional weight loss, pollakiuria, dizziness, dehydration, nausea, and vomiting reported by patients taking empagliflozin among other drugs for type 2 diabetes (Table S3). In addition, the European Medicines Agency recently requested that information on potential risk of toe amputation be included in prescribing information for all SGLT2 inhibitors (84).

Full table
Comparative effectiveness
Low-quality evidence suggests that there are no differences in mortality, morbidity, diabetes control, and serious adverse effects between empagliflozin and metformin (Table 2) (5,31,33,34,37,39,43,46-48,81). Empagliflozin reduces the risk of total non-serious adverse effects when compared with metformin, with 51 avoided adverse events per 1,000 treated (Table 2) (5,31,33,34,37,39,43,46-48,81).

Full table
Very low-quality evidence from a single RCT suggests that empagliflozin decreases HbA1c, the risk of hypoglycemia, and total non-serious adverse effects when compared with glimepiride, at the expense of higher cumulative risk of total combined serious adverse effects (Table 3) (36,42,45). There are no differences in any specific serious harms between empagliflozin and glimepiride (data not shown).

Full table
Low-quality evidence suggests that there are no differences in mortality, morbidity, and serious adverse effects between empagliflozin and linagliptin (Table 4) (5,34,35,46,47). Empagliflozin decreases HbA1c and the risk of total non-serious adverse effects (Table 4) (5,34,35,46,47). Low-quality evidence suggests that there are no differences in mortality, morbidity, and total adverse effects between empagliflozin and sitagliptin (Table 5) (5,32,33,38,40,43,44).

Full table
Full table
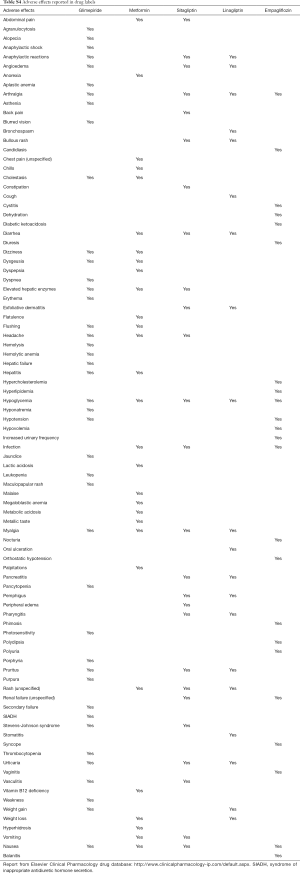
Specific adverse effects differ among examined drugs, according to the labeling information (Table S4). These differences should be taken into account when selecting specific drugs for patients with higher baseline risk of specific harms. Combined drug formulations would likely result in a cumulative increase in the risk of specific adverse effects.
Full table
Post-marketing surveillance suggests that lactic acidosis (6,754 cases), diarrhea (3,774 cases), and acute renal failure (3,754 cases) are the most common adverse effects reported in patients taking metformin among other drugs for type 2 diabetes (Table S3). Hypoglycemia (675 cases), hypoglycemic coma (142 cases), and acute renal failure (126 cases) are the most common adverse effects reported in patients taking glimepiride among other drugs for type 2 diabetes (Table S3). Pancreatitis (328 cases), nausea (216 cases), and rash (178 cases) are the most common adverse effects reported in patients taking linagliptin among other drugs for type 2 diabetes (Table S3). Pancreatitis (2,459 cases), pancreatic carcinoma (1,604 cases), diarrhea (1,175 cases), nausea (1,175 cases), and hypoglycemia (1,163 cases) are the most common adverse effects reported in patients taking sitagliptin among other drugs for type 2 diabetes (Table S3).
Discussion
Our findings that empagliflozin decreases overall and cardiovascular mortality are in concordance with high-quality meta-analyses (5,6). The results are applicable to predominantly white adults with HbA1c 7–10% and established cardiovascular disease. Although the tests for statistical interaction were not significant, ethnic and gender differences in drug benefits need further investigation. Previously published network meta-analyses also suggest that empagliflozin has a favorable benefits-to-harm profile, because it decreases HbA1c and arterial blood pressure without increased risk of hypoglycemia or weight gain (9,85). The evidence regarding the effects of empagliflozin on quality of life and the long-term safety of empagliflozin is insufficient.
Our review also found low-quality evidence that all-cause and cardiovascular mortality and morbidity are comparable after empagliflozin when compared with metformin, glimepiride, linagliptin, or sitagliptin in adults with type 2 diabetes. We downgraded the quality of evidence due to risk of bias in the body of evidence and small number of events in RCTs. We also concluded reporting bias, because reporting of patient morbidity and specific adverse effects was inconsistent across studies, and the results of several completed studies were not available for the analysis. None of the head-to-head RCTs were powered to detect differences in mortality and morbidity. A single RCT suggested reduction in mortality and cardiovascular morbidity after empagliflozin when compared with placebo in adults with established cardiovascular disorder (58,59,68). This pivotal RCT provided only indirect comparative evidence that empagliflozin may be a drug of choice in people with type 2 diabetes and comorbid cardiovascular disorder (58,59,68).
The direct evidence regarding the comparative effectiveness of empagliflozin and other antidiabetic drugs including other SGLT2 inhibitors is insufficient. A recent single RCT demonstrated that injectable liraglutide reduces the risk of major cardiovascular events [hazard ratio (HR) 0.87; 95% CI: 0.78–0.97] and all-cause mortality (HR 0.85; 95% CI: 0.74–0.97) in adults with type 2 diabetes and high cardiovascular risk when compared with placebo (86). Although the RR reduction is larger with empagliflozin, well-designed direct RCTs are needed to conclude the comparative effectiveness of the 2 drugs. Previously published network meta-analyses of intermediate outcomes also suggest that empagliflozin has a favorable benefits-to-harms profile, because it decreases HbA1c without increased risk of hypoglycemia or weight gain (9,15,85).
Our rapid review has several limitations. We did not contact drug manufacturers or principal investigators regarding unpublished or missing data. We do not know how many unregistered, unpublished studies have been conducted. We found no observational studies that provide adjusted for confounding estimates of the comparative effectiveness and safety of empagliflozin and other antidiabetic drugs.
Evidence-based guidelines recommend SGLT2 inhibitors among other available drug classes for adults with type 2 diabetes who could not control diabetes with behavioral changes and metformin (1,2,4). A British guideline based on comprehensive evidence specifies that empagliflozin in combination with metformin should be recommended only to patients who cannot tolerate sulfonylureas or have a high risk of hypoglycemia or its consequences (3).
Future research should examine the long-term comparative benefits and harms of empagliflozin and other drug choices in patient subpopulations by demographics, comorbidities, and concomitant treatments.
Acknowledgements
We would like to thank David R. Goldmann, MD for his contribution to the refinement of the clinical question and review protocol.
This work is supported by Elsevier Evidence-based Medicine Center.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chamberlain JJ, Rhinehart AS, Shaefer CF Jr, et al. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med 2016;164:542-52.
- Fox CS, Golden SH, Anderson C, et al. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Diabetes Care 2015;38:1777-803.
- National Institute for Health and Care Excellence. Type 2 Diabetes in Adults: Management. National Institute for Health and Care Excellence: Clinical Guidelines 2015.
- Qaseem A, Barry MJ, Humphrey LL, et al. Oral Pharmacologic Treatment of Type 2 Diabetes Mellitus: A Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med 2017;166:279-90.
- Monami M, Dicembrini I, Mannucci E. Effects of SGLT-2 inhibitors on mortality and cardiovascular events: a comprehensive meta-analysis of randomized controlled trials. Acta Diabetol 2017;54:19-36.
- Wu JH, Foote C, Blomster J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2016;4:411-9.
- Riggs MM, Staab A, Seman L, et al. Population pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with type 2 diabetes. J Clin Pharmacol 2013;53:1028-38.
- Liakos A, Karagiannis T, Athanasiadou E, et al. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2014;16:984-93.
- Mearns ES, Sobieraj DM, White CM, et al. Comparative efficacy and safety of antidiabetic drug regimens added to metformin monotherapy in patients with type 2 diabetes: a network meta-analysis. PLoS One 2015;10:e0125879.
- Saulsberry WJ, Coleman CI, Mearns ES, et al. Comparative efficacy and safety of antidiabetic drug regimens added to stable and inadequate metformin and thiazolidinedione therapy in type 2 diabetes. Int J Clin Pract 2015;69:1221-35.
- Mondick J, Riggs M, Sasaki T, et al. Mixed-effects modelling to quantify the effect of empagliflozin on renal glucose reabsorption in patients with type 2 diabetes. Diabetes Obes Metab 2016;18:241-8.
- Scheen AJ. Effects of reducing blood pressure on cardiovascular outcomes and mortality in patients with type 2 diabetes: Focus on SGLT2 inhibitors and EMPA-REG OUTCOME. Diabetes Res Clin Pract 2016;121:204-14.
- Shyangdan DS, Uthman OA, Waugh N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open 2016;6:e009417.
- Zhong X, Lai D, Ye Y, et al. Efficacy and safety of empagliflozin as add-on to metformin for type 2 diabetes: a systematic review and meta-analysis. Eur J Clin Pharmacol 2016;72:655-63.
- National Institute for Health and Care Excellence. Canagliflflozin, dapagliflflozin and empagliflflozin as monotherapies for treating type 2 diabetes. Technology appraisal guidance 2016. Available online: nice.org.uk/guidance/ta390
- Higgins J, Green S. editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane book series. London: The Cochrane Collaboration, 2011.
- Slutsky J, Atkins D, Chang S, et al. AHRQ series paper 1: comparing medical interventions: AHRQ and the effective health-care program. J Clin Epidemiol 2010;63:481-3.
- Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 2011;64:1187-97.
- Levine M, Ensom MH. Post hoc power analysis: an idea whose time has passed? Pharmacotherapy 2001;21:405-9.
- Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med 1994;121:200-6.
- Yuan K-H, Maxwell S. On the Post Hoc Power in Testing Mean Differences. Journal of Educational and Behavioral Statistics 2005;30:141-67.
- Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Publication No 10(14)-EHC063-EF 2014. Rockville, MD. Available online: https://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=318
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol 2011;64:1283-93.
- Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013-20.
- Viswanathan M, Berkman ND, Dryden DM, et al. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Aug. Report No.: 13-EHC106-EF.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.
- Grading of Recommendations Assessment Development and Evaluation (GRADE) Working Group. GRADE Handbook. Available online: http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html#h.fueh5iz0cor4
- Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011;64:1311-6.
- Johnston R, Uthman O, Cummins E, et al. Canagliflozin, dapagliflozin and empagliflozin monotherapy for treating type 2 diabetes: systematic review and economic evaluation. Health Technol Assess 2017;21:1-218.
- Jain RK. Empagliflozin/linagliptin single-pill combination therapy for patients with type 2 diabetes mellitus. Expert Opin Pharmacother 2017;18:545-9.
- NCT00789035. 12 Weeks Treatment With 3 Different Doses of BI 10773 in Type 2 Diabetic Patients. 2008.
- NCT00749190. BI 10773 add-on to Metformin in Patients With Type 2 Diabetes. 2008.
- NCT00881530. Empagliflozin (BI 10773) in Type Two Diabetes (T2D) Patients, Open Label Extension. 2009.
- NCT01422876. Efficacy and Safety of Empagliflozin (BI 10773) Linagliptin (BI 1356) Fixed Dose Combination in Treatment naïve and Metformin Treated Type 2 Diabetes Patients. 2011.
- NCT01778049. Linagliptin as Add on Therapy to Empagliflozin 10 mg or 25 mg With Background Metformin in Patient With Type 2 Diabetes. 2013.
- NCT01167881. Efficacy and Safety of Empagliflozin (BI 10773) With Metformin in Patients With Type 2 Diabetes. 2010.
- NCT01368081. Empagliflozin (BI 10773) Comprehensive add-on Study in Japanese Subjects With Type 2 Diabetes Mellitus. 2011.
- NCT01177813. Efficacy and Safety of Empagliflozin (BI 10773) Versus Placebo and Sitagliptin Over 24 Weeks in Patients With Type 2 Diabetes. 2010.
- NCT01719003. Safety and Efficacy Study of Empagliflozin and Metformin for 24 Weeks in Treatment Naive Patients With Type 2 Diabetes. 2012.
- Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 2016;59:1860-70.
- Rosenstock J, Seman LJ, Jelaska A, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab 2013;15:1154-60.
- Ridderstråle M, Svaerd R, Zeller C, et al. Rationale, design and baseline characteristics of a 4-year (208-week) phase III trial of empagliflozin, an SGLT2 inhibitor, versus glimepiride as add-on to metformin in patients with type 2 diabetes mellitus with insufficient glycemic control. Cardiovasc Diabetol 2013;12:129.
- Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care 2013;36:4015-21.
- Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013;1:208-19.
- Ridderstråle M, Andersen KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2014;2:691-700.
- DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 2015;38:384-93.
- Lewin A, DeFronzo RA, Patel S, et al. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care 2015;38:394-402.
- Araki E, Tanizawa Y, Tanaka Y, et al. Long-term treatment with empagliflozin as add-on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab 2015;17:665-74.
- Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME). Cardiovasc Diabetol 2014;13:102.
- Mancia G, Cannon CP, Tikkanen I, et al. Impact of Empagliflozin on Blood Pressure in Patients With Type 2 Diabetes Mellitus and Hypertension by Background Antihypertensive Medication. Hypertension 2016;68:1355-64.
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015;38:420-8.
- Häring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2013;36:3396-404.
- Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014;2:369-84.
- Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 2014;37:1815-23.
- Haering HU, Merker L, Christiansen AV, et al. Empagliflozin as add-on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Res Clin Pract 2015;110:82-90.
- Roden M, Merker L, Christiansen AV, et al. Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug-naive patients with type 2 diabetes: a double-blind extension of a Phase III randomized controlled trial. Cardiovasc Diabetol 2015;14:154.
- Chilton R, Tikkanen I, Cannon CP, et al. 4B.02: the sodium glucose cotransporter 2 inhibitor empagliflozin reduces blood pressure and markers of arterial stiffness and vascular resistance in type 2 diabetes. J Hypertens 2015;33 Suppl 1:e53.
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 2016;375:323-34.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015;373:2117-28.
- Kanada S, Koiwai K, Taniguchi A, et al. Pharmacokinetics, pharmacodynamics, safety and tolerability of 4 weeks' treatment with empagliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2013;4:613-7.
- Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin as Add-on Therapy to Pioglitazone With or Without Metformin in Patients With Type 2 Diabetes Mellitus. Clin Ther 2015;37:1773-88.e1.
- NCT01370005. 12 Week Efficacy and Safety Study of Empagliflozin (BI 10773) in Hypertensive Patients With Type 2 Diabetes Mellitus. 2011.
- NCT01011868. Efficacy and Safety of BI 10773 in Combination With Insulin in Patients With Type 2 Diabetes. 2009.
- NCT01164501. Efficacy and Safety of Empagliflozin (BI 10773) in Patients With Type 2 Diabetes and Renal Impairment. 2010.
- NCT01210001. Efficacy and Safety of Empagliflozin (BI 10773) in Type 2 Diabetes Patients on a Background of Pioglitazone Alone or With Metformin. 2010.
- NCT01289990. Safety and Efficacy of Empagliflozin (BI 10773) and Sitagliptin Versus Placebo Over 76 Weeks in Patients With Type 2 Diabetes. 2011.
- NCT01306214. Safety and Efficacy of BI 10773 as add-on to Insulin Regimen in Patients With Type 2 Diabetes Mellitus. 2011.
- NCT01131676. BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME). 2010.
- NCT01159600. Efficacy and Safety Study With Empagliflozin (BI 10773) vs. Placebo as add-on to Metformin or Metformin Plus Sulfonylurea Over 24 Weeks in Patients With Type 2 Diabetes. 2010.
- Kadowaki T, Haneda M, Inagaki N, et al. Efficacy and safety of empagliflozin monotherapy for 52 weeks in Japanese patients with type 2 diabetes: a randomized, double-blind, parallel-group study. Adv Ther 2015;32:306-18.
- NCT01193218. Empagliflozin (BI 10773) Dose Finder Study in Japanese Patients With Type 2 Diabetes Mellitus. 2010.
- NCT01649297. A 16 Weeks Study on Efficacy and Safety of Two Doses of Empagliflozin (BI 10773) (Once Daily Versus Twice Daily) in Patients With Type 2 Diabetes Mellitus and Preexisting Metformin Therapy. 2012.
- Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. European heart journal 2016;37:1526-34.
- Zinman B, Inzucchi SE, Lachin JM, et al. Empagliflozin and Cerebrovascular Events in Patients With Type 2 Diabetes Mellitus at High Cardiovascular Risk. Stroke 2017;48:1218-25.
- Zhao X, Cui Y, Zhao S, et al. Pharmacokinetic and Pharmacodynamic Properties and Tolerability of Single- and multiple-dose Once-daily Empagliflozin, a Sodium Glucose Cotransporter 2 Inhibitor, in Chinese Patients With Type 2 Diabetes Mellitus. Clin Ther 2015;37:1493-502.
- Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther 2013;4:331-45.
- Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol 2015;14:11.
- NCT01947855. Post Prandial Glucose (PPG) Study of Empagliflozin in Japanese Patients With Type 2 Diabetes Mellitus. 2013.
- NCT01316341. Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of Empagliflozin in Chinese Female and Male Patients With Type 2 Diabetes Mellitus. 2011.
- NCT01924767. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Multiple Rising Doses of BI 10773 Tablets. 2007.
- NCT00558571. 4 Week Treatment With Three Oral Doses of BI 10773 in Patients With Type 2 Diabetes. 2008.
- Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks' treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 2013;15:613-21.
- NCT00885118. 4 Weeks Treatment With Empagliflozin (BI 10773) in Japanese Type 2 Diabetic Patients (T2DM). 2009.
- European Medicines Agency (EMA). SGLT2 inhibitors: information on potential risk of toe amputation to be included in prescribing information. EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) 2017 EMA/118223/2017.
- Gross JL, Kramer CK, Leitao CB, et al. Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: A network meta-analysis. Ann Intern Med 2011;154:672-9.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016;375:311-22.


