Outcomes of a newer-generation cementless total knee arthroplasty design in patients less than 50 years of age
Introduction
The incidence of total knee arthroplasty (TKA) in younger patient populations has increased over the past several years, and over the next decade, patients younger than 65 years of age are expected to account for 55% of these procedures performed in the United States (1). This growing population is likely a result of the growing incidence of obesity and the expanded indications for this procedure (2-5). Cemented fixation has been the most commonly used method for TKA in younger patients with high 10- to 18-year survivorship (94% and 98%) (6-9). However, others have demonstrated lower implant survivorships, potentially resulting from these patients having higher activity levels and placing greater stress on the implants (6,10-12). Furthermore, there have been concerns regarding cemented implants, particularly in younger patients, in terms of problems at the bone-cement interface such as osteolysis, bone resorption, and aseptic loosening (13,14).
Cementless implants were developed as a method to potentially preserve the native bone stock and improve the implant longevity (15-18). While the early implants were associated with a high failure rate due to aseptic loosening (18-20), advances in materials and designs led to the development of newer implants, which consist of bioactive surface coatings that allow for improved fixation (21). Recent studies have demonstrated excellent outcomes with the newer generation cementless TKA implants (21-24). However, there is a paucity of literature that has reported on the outcomes of newer cementless TKA designs in patients younger than 50 years, a much more active cohort than the traditional TKA patients.
Therefore, the purpose of this study was to evaluate: (I) implant survivorship; (II) functional outcomes and complications; and (III) radiographic outcomes in patients who were less than 50 years of age and underwent cementless TKA.
Methods
Patient selection
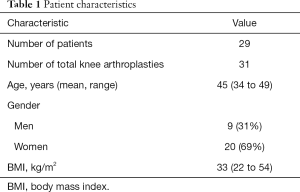
After institutional review board approval was obtained, all patients who were less than 50 years of age, who underwent a primary TKA at a single, high-volume institution (Mount Sinai Beth Israel, New York, New York, USA) from June 2008 to May 2014 were identified from a prospectively collected database. We included patients who underwent TKA for any reason and had at least 2 years of follow-up. We excluded all revision cases and patients who had follow-up less than 2 years. A total of 29 patients (31 knees) who had a mean follow-up of 4 years (range, 2 to 6 years) were analyzed (Table 1). The cohort included 20 women (69%) and 9 men (31%), who had a mean age of 45 years (range, 34 to 49 years), and a mean body mass index of 33 kg/m2 (range, 22 to 54 kg/m2). There were 24 patients who had osteoarthritis (77%), 5 patients who had osteonecrosis (16%), and 2 patients who had rheumatoid arthritis (7%).
Full table
TKA implants
From June 2008 to June 2013, the cementless TKA design that patients received was a beaded, periapatite-coated (PA) femoral component and a cobalt-chrome tibial baseplate (Triathlon Total Knee System; Stryker Orthopaedics, Mahwah, New Jersey, USA) (n=22 knees). Multiple layers of cobalt-chromium beads were incorporated in the implant, forming a 1.5 mm thick coating, with an average pore size of 425 µm and a porosity of 35%. To provide a 3-dimensional coating, periapatite, which is a highly crystalline solution form of hydroxyapatite, was used. The femoral component was an open posterior-stabilized box with medial and lateral pegs.
A highly porous titanium coated baseplate (Triathlon Tritanium tibial baseplate; Stryker Orthopaedics, Mahwah, New Jersey, USA) became available in June 2013 and was used thereafter (n=9 knees). A 3-dimensional modeling and analytical technology (SOMA; Stryker Orthopaedics, Mahwah, New Jersey, USA) was used to design the baseplate, to provide better anthropometric sizing by using an extensive computed tomography scan-based database to improve fit and optimize fixation of the tibial baseplate pegs. Since it identified the optimal areas for bone fixation, instead of screws, a delta keel and 4 peg system was employed.
In October 2014, the beaded PA-coated patellar component was replaced by a highly porous titanium-backed patellar component that had 3 pegs (Triathlon Tritanium patella; Stryker Orthopaedics, Mahwah, New Jersey, USA).
Surgical procedure and rehabilitation
All of the procedures were performed using a midline skin incision, and a minimally invasive mid-vastus approach to the knee joint. Gap-balancing techniques were used to equalize the flexion and extension gaps. If bone defects or cysts were identified, they were filled with autologous bone, and a 2 mm drill bit was used to drill sclerotic areas of bone. After implantation, range of motion, stability, and patella tracking were evaluated. Routine closure was performed.
A standard, accelerated postoperative physical therapy program with full weight-bearing and range-of-motion exercises was started prior to hospital discharge.
Follow-up
Postoperatively, patients were assessed at 6-weeks, 3-month, 1-year, and then annually. At each follow-up visit, the Knee Society pain and function scores (25) were collected, and the new Knee Society Radiographic Evaluation and Scoring System (26) was used to evaluate the postoperative radiographs. All of the radiographs were performed by 1 of 2 experienced technicians, yielding uniform results without the use of fluoroscopic positioning. Preoperative femoro-tibial angle on standing antero-posterior radiographs showed 19 varus knees (mean of 7.5 degrees; range, 5 to 30 degrees), 8 valgus knees (mean of 12.5 degrees; range, 5 to 30 degrees), and 4 neutral aligned knees (less than 5 degrees of deformity). Post-operative complications were assessed using Standardized List and Definitions of The Knee Society (bleeding, wound complications, thromboembolic disease, neural deficit, vascular injury, medial collateral ligament injury, instability, malalignment, stiffness, deep periprosthetic joint infection, periprosthetic fracture, extensor mechanism disruption, patellofemoral dislocation, tibiofemoral dislocation, bearing surface wear, osteolysis, implant loosening, implant fracture or tibial insert dissociation, reoperation, revision, readmission, death) (27). Any radiographic signs of component loosening, radiolucency, gap formation between the implant and the bone, subsidence, or reactive changes were documented.
Statistical analysis
Descriptive statistics were used to analyze the mean and ranges for the continuous variables. Additionally, a Kaplan-Meier survival analysis, with 95% confidence intervals, was performed to determine the implant survivorship, with the endpoint being revision for any reason. All statistical analyses were performed using SPSS version 23 (IBM Corporation, Armonk, New York, USA).
Results
Survivorship
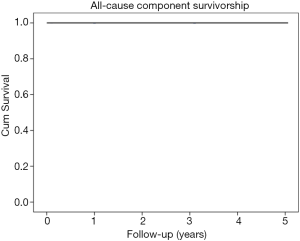
This cohort of patients had aseptic survivorship of 100% (Figure 1). There were no aseptic failures noted, and there were no revisions surgeries performed as of the latest follow-up visit. There were no deep peri-prosthetic infections in this cohort of patients, thus the overall survivorship was also 100%.
Functional outcomes and complications
At the latest follow-up, the mean Knee Society pain score was 92 points (range, 80 to 95 points) and the mean Knee Society function score was 84 points (range, 70 to 90 points). Additionally, at the latest follow-up, the mean knee extension was 1 degree (range, 0 to 5 degrees) and the mean knee flexion was 125 degrees (range, 95 to 140 degrees). As of the latest clinical follow-up, none of the patients had suffered from any postoperative complications.
Radiographic outcomes
At the latest follow-up, the radiographic evaluation revealed that there was no evidence of component loosening, progressive radiolucency, or reactive changes for any of the prosthetic components. All components appeared stable and no subsidence was noted when compared to previous radiographs.
Discussion
TKA with cementless fixation was developed to decrease cement-related complications, to potentially preserve the native bone stock, and to prolong implant survivorship (13-18). Even though the original designs were associated with early failures, innovations in technology led to newer implants and biomaterials that accelerate implant osseointegration, which could eventually lead to improved long-term survivorship of cementless implants. Moreover, with the growing number of younger, more active patients requiring TKAs, cementless fixation may be the best modality that is most suitable for those who have an active lifestyle; thereby, decreasing the risk for revision surgery. The results of the present study found that at mean 4-year follow-up, patients less than 50 years of age who underwent cementless TKA had a 100% survivorship implant survivorship and excellent functional outcomes scores and range-of-motion.
There were several limitations of the present study. We only assessed a cohort of patients who received cementless TKAs, and did not compare the outcomes to cemented TKAs. Additionally, this study had a small sample size and was performed at a single institution; however, if future, multi-center studies are performed that follow a similar protocol, this information could be generalized to the whole population. Furthermore, this study reported on the early outcomes, and since this is a young population of patients, a much longer follow-up is required in order to determine the true longevity of these implants. Despite these limitations, the results of the present study demonstrated excellent early results, which may lead to successful long-term outcomes of cementless TKAs in younger patients.
Several studies have demonstrated excellent outcomes in younger patients after undergoing cementless TKA using newer-generation implants. Tai and Cross (28) prospectively followed 92 patients (118 knees) who were 55 years or younger and underwent cementless TKA with hydroxyapatite-coated implants, and had a mean follow-up of 8 years (range, 5 to 12 years). They reported that the overall survival rate at 12 years was 97.5%, with 2 patients who developed aseptic loosening and underwent revision surgery. Similarly, in a prospective, randomized, double blinded study, Lizaur-Utrilla et al. (29) performed 45 cementless and 48 cemented TKAs in patients who were less than 55 years, and had a mean follow-up of 7 years (range, 5 to 12 years). They reported that the 9-year survivorship for aseptic failure was 94% in the cementless group and 90% in the cemented group; 1 patient in the cementless and 4 patients in the cemented groups underwent revision for aseptic loosening. Also, at the latest follow-up, the cementless group had significantly better knee (94 vs. 89 points, P=0.022) and pain scores (47 vs. 44 points, P=0.024) compared to the cemented group. Kamath et al. (30) reported on 100 patients (100 knees) who were less than 55 years and received cementless TKAs, and 312 patients (312 knees) who had a mean age of 63 years who received cemented TKAs, and had at least a 5-year follow-up. They determined that there were no differences between the cementless and cemented TKAs in terms of Knee Society knee scores (95 vs. 91 points, P>0.05) or functional scores (88 vs. 86 points, P>0.05). While there were 2 cases of aseptic loosening in the cemented group, there were 3 failures in the cementless group, none of which were related to implant fixation.
Although the newer studies showed favorable results in these implants, studies on the older designs have reported less than satisfactory outcomes with cementless TKAs. These include reports by Moran et al. (31) as well as Meneghini and de Beaubien (32), who reported a failure rate of 19% and 8% using older generation implants.
In conclusion, this study demonstrated that younger patients who are less than 50 years had excellent midterm implant survivorship and functional outcomes. Longer follow-up of this patient cohort will continue, and will allow us to make conclusions on the long-term outcomes. As the population of younger patients undergoing TKA continues to grow, cementless implants may be the appropriate design to ensure long-term durability and survivorship.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Mont is a paid consultant for Stryker and receives research support and IP royalties from Stryker. Dr. Harwin is a paid consultant and paid presenter or speaker for Stryker. He receives IP royalties and holds stock or stock options from Stryker. The other authors have no conflicts of interest to declare.
Ethical Statement: Institutional review board approval was obtained.
References
- Kurtz SM, Lau E, Ong K, et al. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res 2009;467:2606-12. [Crossref] [PubMed]
- Apold H, Meyer HE, Nordsletten L, et al. Weight gain and the risk of knee replacement due to primary osteoarthritis: a population based, prospective cohort study of 225,908 individuals. Osteoarthr Cartil 2014;22:652-8. [Crossref] [PubMed]
- Issa K, Pierce TP, Scillia AJ, et al. Midterm Outcomes Following Total Knee Arthroplasty in Lupus Patients. J Arthroplasty 2016;31:655-7. [Crossref] [PubMed]
- Palmer DH, Mulhall KJ, Thompson CA, et al. Total knee arthroplasty in juvenile rheumatoid arthritis. J Bone Joint Surg Am 2005;87:1510-4. [PubMed]
- Goodman SM, Springer B, Guyatt G, et al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Rheumatol 2017;69:1538-51. [Crossref] [PubMed]
- Meftah M, White PB, Ranawat AS, et al. Long-term results of total knee arthroplasty in young and active patients with posterior stabilized design. Knee 2016;23:318-21. [Crossref] [PubMed]
- Crowder AR, Duffy GP, Trousdale RT. Long-term results of total knee arthroplasty in young patients with rheumatoid arthritis. J Arthroplasty 2005;20:12-6. [Crossref] [PubMed]
- Ranawat CS, Padgett DE, Ohashi Y. Total knee arthroplasty for patients younger than 55 years. Clin Orthop Relat Res 1989.27-33. [PubMed]
- Diduch DR, Insall JN, Scott WN, et al. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am 1997;79:575-82. [Crossref] [PubMed]
- Paxton EW, Namba RS, Maletis GB, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am 2010;92 Suppl 2:117-32. [Crossref] [PubMed]
- Duffy GP, Crowder AR, Trousdale RR, et al. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty 2007;22:67-70. [Crossref] [PubMed]
- Harrysson OL, Robertsson O, Nayfeh JF. Higher cumulative revision rate of knee arthroplasties in younger patients with osteoarthritis. Clin Orthop Relat Res 2004.162-8. [Crossref] [PubMed]
- Kim YH, Park JW, Lim HM, et al. Cementless and cemented total knee arthroplasty in patients younger than fifty five years. Which is better? Int Orthop 2014;38:297-303. [Crossref] [PubMed]
- Naudie DD, Ammeen DJ, Engh GA, et al. Wear and osteolysis around total knee arthroplasty. J Am Acad Orthop Surg 2007;15:53-64. [Crossref] [PubMed]
- Chong DY, Hansen UN, van der Venne R, et al. The influence of tibial component fixation techniques on resorption of supporting bone stock after total knee replacement. J Biomech 2011;44:948-54. [Crossref] [PubMed]
- Brown TE, Harper BL, Bjorgul K. Comparison of cemented and uncemented fixation in total knee arthroplasty. Orthopedics 2013;36:380-7. [Crossref] [PubMed]
- Dalury DF. Cementless total knee arthroplasty: current concepts review. Bone Joint J 2016;98-B:867-73. [Crossref] [PubMed]
- Meneghini RM, Hanssen AD. Cementless fixation in total knee arthroplasty: past, present, and future. J Knee Surg 2008;21:307-14. [Crossref] [PubMed]
- Mont MA, Pivec R, Issa K, et al. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg 2014;27:369-76. [PubMed]
- Cherian JJ, Banerjee S, Kapadia BH, et al. Cementless total knee arthroplasty: a review. J Knee Surg 2014;27:193-7. [Crossref] [PubMed]
- Harwin SF, Patel NK, Chughtai M, et al. Outcomes of Newer Generation Cementless Total Knee Arthroplasty: Beaded Periapatite-Coated vs Highly Porous Titanium-Coated Implants. J Arthroplasty 2017;32:2156-60. [Crossref] [PubMed]
- Newman JM, Khlopas A, Chughtai M, et al. Cementless Total Knee Arthroplasty in Patients Older Than 75 Years. J Knee Surg 2017;30:930-5. [Crossref] [PubMed]
- Nam D, Kopinski JE, Meyer Z, et al. Perioperative and Early Postoperative Comparison of a Modern Cemented and Cementless Total Knee Arthroplasty of the Same Design. J Arthroplasty 2017;32:2151-5. [Crossref] [PubMed]
- Kwong LM, Nielsen ES, Ruiz DR, et al. Cementless total knee replacement fixation: a contemporary durable solution--affirms. Bone Joint J 2014;96-B:87-92. [Crossref] [PubMed]
- Insall JN, Dorr LD, Scott RD, et al. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 1989.13-4. [PubMed]
- Meneghini RM, Mont MA, Backstein DB, et al. Development of a Modern Knee Society Radiographic Evaluation System and Methodology for Total Knee Arthroplasty. J Arthroplasty 2015;30:2311-4. [Crossref] [PubMed]
- Healy WL, Della Valle CJ, Iorio R, et al. Complications of total knee arthroplasty: standardized list and definitions of the Knee Society. Clin Orthop Relat Res 2013;471:215-20. [Crossref] [PubMed]
- Tai CC, Cross MJ. Five- to 12-year follow-up of a hydroxyapatite-coated, cementless total knee replacement in young, active patients. J Bone Joint Surg Br 2006;88:1158-63. [Crossref] [PubMed]
- Lizaur-Utrilla A, Miralles-Muñoz FA, Lopez-Prats FA. Similar survival between screw cementless and cemented tibial components in young patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2014;22:1585-90. [Crossref] [PubMed]
- Kamath AF, Lee GC, Sheth NP, et al. Prospective results of uncemented tantalum monoblock tibia in total knee arthroplasty: minimum 5-year follow-up in patients younger than 55 years. J Arthroplasty 2011;26:1390-5. [Crossref] [PubMed]
- Moran CG, Pinder IM, Lees TA, et al. Survivorship analysis of the uncemented porous-coated anatomic knee replacement. J Bone Joint Surg Am 1991;73:848-57. [Crossref] [PubMed]
- Meneghini RM, de Beaubien BC. Early failure of cementless porous tantalum monoblock tibial components. J Arthroplasty 2013;28:1505-8. [Crossref] [PubMed]