Digital chest tomosynthesis: the 2017 updated review of an emerging application
Introduction
Digital tomosynthesis (DT) is a technology that provides some of the tomographic benefits of computed tomography (CT) but at reduced radiation dose and cost. Tomosynthesis stems from the older technique of geometric tomography which was developed in the 1930’s by the Italian radiologist Alessandro Vallebona (1), and it has mostly fallen out in favour of chest imaging owing to positioning difficulty, high radiation dose, and residual blur from out of plane structures. After more than 100 years, conventional chest radiography (CR) is still the main examination in chest radiology. The term tomosynthesis was defined by combining the Greek words “tomos” (a section, a slice, cutting) and “synthesis” (a process) (2). This technique overcomes the difficulties of geometric tomography by permitting reconstruction of many slices in the patient from a single low-dose acquisition of image data. It uses a conventional Roentgen’s tube and a digital detector to acquire a set of projection images that are combined to synthesise any plane in the patient (3). Unlike conventional tomography, tomosynthesis is not limited to reconstruction of a single plane, but rather can generate an arbitrary number of slice images throughout the entire volume of the patient (4). In this way, tomosynthesis addresses one of the primary weakness of conventional single-projection X-ray imaging, the superposition of objects in the image, which may result in the obscuring of an object of interest or production of structure that simulates a disease (5). Despite some early successful proofs of concept, tomosynthesis has only recently become practical as a clinical imaging modality. This technique is now under investigation for application to some clinical detection tasks (6) and has recently been implemented in commercial devices to provide images of chest, abdominal, breast, head, and neck (7). In fact, tomosynthesis provides improved visibility of anatomical structures, for example in the chest it’s possible to visualise lungs, airways and ribs with more accuracy (8). In this review, we will focus on pulmonary nodule detection and show characteristics, advantages, and limitations of tomosynthesis.
Background
CR is a basic technique for the investigation of pulmonary disease, of which lung cancer is the most feared one (9).
Lung cancer, in fact, is the leading cause of cancer-related deaths in most of the western and developing countries. There are three main categories of lung cancer. Non-small cell lung cancer (NSCLC) is the most common (85% of people diagnosed with lung cancer each year). Less common than NSCLC, small cell lung cancer (SCLC) is only diagnosed in 10–15% of people diagnosed with lung cancer but is more aggressive than NSCLC and can spread quickly (10). Finally, lung carcinoid tumour is the least common of the three primary types of lung cancer. Less than 5% of lung cancers diagnosed each year are lung carcinoid tumours. These slow-growing tumours rarely spread. Each type and stage of lung cancer have a different survival rate. The overall 5-year survival rate for NSCLC (all stages combined) is roughly 18 percent. The overall 5-year survival rate for SCLC is only about six percent (11,12).
About 85% to 90% of patients with lung cancer have had direct exposure to tobacco. Many tobacco-related carcinogens have been identified (13). Although the smoking prevalence has decreased, approximately 37% of adults are current or former smokers. It is well known that the incidence of lung cancer increases with age. Increasing age and cumulative exposure to tobacco smoke are the two most common risk factors for lung cancer (10).
The radiologist analysing a chest radiograph and detecting an unexplained opacity in the lung is dealing with a diagnostic dilemma since many nodules do not represent lung malignancies (14). Nowadays, CT is recognised as the best technique for the lung cancer diagnosis, and low-dose CT (LDCT) is considered the actual standard for lung cancer screening (early detection). However, due to some limitation of CT (mainly regarding high radiation exposure, high cost and motion artefacts), new techniques such as DT have been inspected (15).
Technical characteristics
Acquisition technologies
During a tomosynthesis image acquisition, both Roentgen’s tube and a large digital flat-panel detector are moved over a pre-defined trajectory driven by a computer-controlled machine mover. The first item to consider when describing tomosynthesis imaging is the geometry of motion of the tube and/or detector. There are three basic motion geometries: parallel path, partial isocentric motion and full isocentric motion (Figure 1).
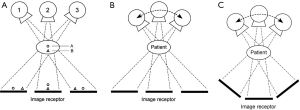
In the first one, the tube moves in a plane parallel to the detector plane. In the second one, the indicator remains immobile, and the Roentgen’s tube moves in an arc above the detector while in the latter one the tube and detector are fixed rigidly to each other and move in tandem in a circular path around the patient. The parallel-path motion is typically used in the chest and abdominal tomosynthesis.
Geometrical parameters include angular range, angular step size and projection density. The angular range of a Roentgen’s tube rotation is defined as the length of the whole arc above the pivot centre, described by an X-ray machine from the first to the last measured projection position. The angular range of clinical applications is typically between 20° and 50°. The number of projections is the number of measured X-ray images acquired over the angular range. In clinical applications, it is generally between 10° and 30°. The angular step size is defined as the total angular range divided by the number of projections and is characterised by the Roentgen’s tube movement from the current to the next measured projection position (3).
Imaging process
The function of DT is to reconstruct an unknown distribution of X-ray attenuation coefficients within the imaged object based on a set of measured projection images, i.e., to solve an inverse problem. The result of the reconstruction is a stack of slices, which is parallel to the detector. The comparison between a projection image and a reconstructed slice of a hand show that the second one has a higher contrast and more details when compared to the first one.
Although tomosynthesis is a volumetric imaging technique and provides spatial information about the location of structures, the complete three-dimensional information about the object cannot be reconstructed. Therefore, an accurate image reconstruction in DT becomes a challenging task. Tomosynthesis images typically contain limited angle artefacts and have an anisotropic resolution.
Artefacts appear as multiple blurred copies of structures which are located above or below the plane of interest. They might hide or corrupt the appearance of structures of interest within the object. In the past, several investigators explored methods to reduce the blur artefacts associated with tomosynthesis imaging, and these deblurring algorithms made tomosynthesis suitable for consideration in a wider range of clinical applications (5).
Algorithms for the reconstruction
The choice of reconstruction algorithm and the optimisation of reconstruction parameters greatly influence the properties of the reconstructed images. Various reconstruction approaches to improve image quality and reduce artefacts in tomosynthesis have been proposed so far.
Historically the first reconstruction algorithm is a straightforward back projection (BP), also known as “Shift & Add”. This method brings in-plane objects in focus while blurring out-off-plane features. It is often used due to its relatively straightforward implementation and minimal computational power requirements, but it results in pictures with reduced contrast and necessitates post-processing of pictures. Thus, the conventional “Shift & Add” algorithm suffers from considerable problems in this field of use. Another method to reconstruct tomosynthesis slice, called tuned aperture CT, is basically “Shift & Add” with fiducial markers and it allows the acquisition of images at random angles and orientations.
The two deblurring algorithms that have received the most attention in recent years are matrix inversion tomosynthesis (MITS) and filtered BP (FBP). While MITS solves for the out-of-plane blur using the public blurring functions of all other planes when a given plane is reconstructed (16), the deblurring algorithm that is used today by many tomosynthesis investigators is FBP anyway. FBP is well known from decades of work in CT and, like MITS is a computationally fast algorithm. FBP consists of low-pass filters which are used in the spatial frequency domain to compensate for an incomplete or non-uniform sampling of the tomography acquisition in the space domain to suppress high frequencies (17-19).
Iterative algorithms are another type of reconstruction in which the slice at different depth are iteratively updated until a certain stopping criterion is met. This technique can be subdivided into two classes: algebraic and statistical reconstruction. Algebraic methods express the reconstruction problem as a system of linear equations under defined and without a unique solution. Statistical reconstruction considers Poisson noise model and maximises a likelihood function. An advantage of this approach is that all the components of the imaging system can be shaped, but the downside is that the technique is iterative and quite computationally intensive. For these reasons, the FBP method is considerate superior to find masses and small calcifications.
However, it is important to remark that all algorithms suffer from the incompleteness of the tomosynthesis projection dataset and none can provide artifact-free images with perfect image quality. A detailed comparison of Reconstruction algorithms for tomosynthesis can be found in Zhang et al. (20).
Effect of acquisition parameters
Another option to improve tomosynthesis performance regarding image quality is optimisation of the device hardware. It comprises an examination of the impact of the acquisition parameters and geometry. Increasing the number of measured projections improves image quality, but this effect tends to have boundaries. The angular range plays a more significant role. An increase in the angular range always advances image quality. The main conclusion is that the more data acquired about the object, the better the image quality. The most significant limitation is that the patient dose should not increase excessively. This issue stimulates the search for alternative acquisition schemes enabling the purchase of supplementary information about the object without increasing the radiation dose. This could be achieved with the X-ray source in two perpendicular directions, a solid angle tomosynthesis, or by replacing the Roentgen’s tube with an X-ray source array using specially designed carbon nanotubes (CNT) (21). In this way, additional angulated projections can be acquired, which leads to a superior image quality. The optimal parameters and geometry must be adapted for each application.
Image analysis
The results of tomosynthesis’ studies indicate impaired visibility of small anatomical structures in DT when performing a head-to-head comparison with CT. Furthermore, the profile in DT was dependent on both anatomical location and observer experience. The profile for DT was significantly higher in the central and peripheral lateral regions than that in the anterior and posterior outer regions. Besides, motion artefacts and limited depth resolution as leading causes of reduced visibility in DT. Since outlines become more blurred (contrast decreases), the structures away from the focus plane are less disturbing (14). The most experienced observer had significantly higher visibility ratings, compared with the less experienced observers (22).
Digital chest tomosynthesis (DCT)
DCT, as we have seen, is a promising technology which overcomes some difficulties and brings some advantages by permitting the reconstruction of various image slices from a single low-dose acquisition of image data.
Compared to standard radiography, DCT has several advantages like improved lesion detection due to a reduction in anatomical noise or composite artefact, better depth localisation and contrast resolution. Recent reports have shown that the use of tomosynthesis, as an alternative of CR, leads to considerable improvement in diagnostic information without an increase in radiation dose (Figure 2).
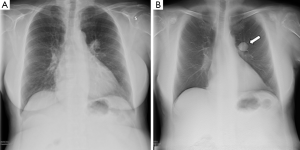
In fact, if posterior-anterior chest roentgenogram would result in a radiation dose to the lungs of 0.01 mSv and a lateral chest roentgenogram of 0.15 mSv (23), tomosynthesis related dose is approximately 0.1–0.2 mSv (24,25). Moreover, when compared to CT, the advantages of DCT consist in a pronounced reduction of cost, in addition to reduced radiation dose to patients [4–8 mSv in chest CT (26) and 1.5 mSv in LDCT (27)].
DCT is emerging as a promising technology for the diagnosis of equivocal or suspected pulmonary lesions on CR. Once DCT identifies chest opacity as probably being a pulmonary lesion, a CT scan is required for further evaluation, classification, and staging (28).
The use of DCT would entail less waiting time for the workup of the suspected lung lesion that then turns out to be benign, non-pulmonary, or a false positive nodule. Prospectively, DCT could spare further CR and could replace CT scan in the characterization of incidental chest lesions identified by conventional radiography (6).
In Kim et al. phantom study (29), results that DCT was superior to radiography and dual-energy subtraction (DES) for detection of smaller solid simulated pulmonary nodules (SPN). DES is another technique that takes advantage of differences in the degree to which body tissues attenuate low- and high-energy (measured in kilo-electron volts) photons (30). These differences are used to generate tissue-selective images. In this study lung fields were divided on CR into eight regions based on anatomy, these areas were classified into two groups of the danger zone and non-danger zone. Metrics frequently used in the free-response task were: the lesion localisation fraction (LLF), which is the number of lesion localisations divided by the total number of lesions; and non-lesion localisation fraction (NLF), which is the number of non-lesion localisations divided by the total number of cases. Using DCT for the detection of smaller SPN (5–8 mm), observer averaged LLFs were significantly (P=0.004) higher in the non-danger zones than those of the danger zones. However, for the detection of larger SPN (10 and 12 mm), observer-averaged LLFs were equal t=1 (perfect) regardless of nodule location. A detailed comparison of the observer-averaged LLFs of each technique according to subdivided location and size of SPN demonstrated results as follows: DCT was not superior to radiography and DES for the detection of every size of SPN in the retro-diaphragmatic region (danger zone). In fact, in comparison with radiography, DCT was significantly superior to detect smaller SPN (5 and 8 mm) in the apical and paramediastinal regions (danger zones) and the lateral pulmonary region (non-danger zone). However, DCT was significantly superior not only to detecting smaller SPN (5 and 8 mm) but also larger ones (10 and 12 mm) in the paramediastinal region (danger zone). Otherwise, DCT was not superior to radiography to detect more substantial SPN (10–12 mm) in the apical region (danger zone) and the lateral pulmonary region (non-danger zone). In comparison with DES, DCT was not superior to detect 8-mm SPN in the apical region (danger zone) providing the results like those of the radiography.
Choo et al. (31) compared DCT and CR using CT as a reference and considered the validity and reliability of both techniques for the detection of airway lesions. The sensitivity of DCT results higher than CR. Moreover, even the diagnostic accuracy of the first one performs better than the second one. However, specificity was not significantly different. About reliability, receiver operating characteristic (ROC) analyses using the scores of detections confidence were performed, and a significant difference between the area under the curve (AUC) value of two modalities came from the significantly higher sensitivity of DCT. In most cases, DCT demonstrated airway lesions are precisely providing more accurate information to evaluate the extent and severity of airway lesions.
The advantage of DCT was slightly more apparent for noncalcified nodules. The sensitivity of each modality to classify the calcification in identified nodules was also investigated (8).
Here’s a list of the most promising use of DCT.
DCT as a lung screening tool
Recently, LDCT screening for lung cancer has been reported to reduce lung cancer mortality (27), and to date, it is the only recommended screening tool for lung cancer. The U.S. Preventive Services Task Force (USPSTF) recommends yearly lung cancer screening with LDCT for people who: have a history of heavy smoking (history of 30 pack years or more); smoke now or have quit within the past 15 years, are between 55 and 80 years old (32).
While LDCT has shown to improve survival rates, other methods of detecting lung cancer early that use less radiation and are less expensive than CT are desired (33). In an observational study (15) involving nearly 2,000 subjects, the percentage of lung nodules and lung cancer detected with tomosynthesis was comparable to that reported for LDCT. These findings put tomosynthesis in the spotlight as a potentially lower-dose, lower-cost option for lung cancer screening.
DCT in the assessment of solitary pulmonary nodules detected with radiography
Another common clinical issue is the accidental detection of a solitary pulmonary nodule. According to the definition, a solitary nodule is defined as a detached, with margin, rounded opacity <3 cm in diameter enclosed in lung parenchyma, not reaching the hilum or the mediastinum, without adenopathies, atelectasis, or pleural effusion (34). Detection of pulmonary nodules is a challenging task in thoracic imaging because these lesions are frequently small and show poor relation about the surrounding anatomic structures.
Chest radiographs of the patient allow assessment of the growth rate, and it refers to the doubling time of a nodule, i.e., the time necessary for the nodule’s volume to double. On chest radiographs, a nodule appears as a 2-dimensional representation of a 3-dimensional structure. The volume of a sphere equals 4/3PR3. Therefore a 26% increase in diameter on a chest radiograph represents one doubling in size (35). DCT is an alternative that provides 3-dimensional information, and it can be used as a complementary technique in the diagnosis of equivocal or suspected pulmonary lesions on CR. DCT could replace many CT confirmatory scanning now needed after doubtful chest radiographs.
DCT as detection tool for secondary lung cancer
Secondary lung tumours are neoplasms that spread from a primary lesion. Practically any cancer can spread to the lungs, but the neoplasms commonly do include bladder, colon, breast, prostate, sarcoma, Wilms tumour, and neuroblastoma. DCT could be an ideal imaging tool for this problem; alternating CT scan and DCT interval examinations may become an optimal solution to reduce costs and radiation risk (36). DCT could be quickly introduced in the routine diagnostic workflow as a case-solving technique in oncologic patients with suspected or ambiguous pulmonary lesions on radiography (37).
Limitations
As the projection images in tomosynthesis are not acquired over 360°, the depth resolution in reconstructed section images is limited. Thus, the isotropic resolution possible with modern CT equipment cannot be achieved with tomosynthesis. This prevents the complete removal of superimposed tissue in tomosynthesis. Instead, anatomy surrounding a plane of interest will to some extent always be present in each section image, and a given object will show up in more than one section image. The little resolution in the z direction in comparison with CT makes it difficult to correctly localise a structure in this axis, which may result in pitfalls for many clinical tasks. Also, the limited depth resolution may lead to artefacts. The combination of the facts that the acquisition of the original of projection images takes several seconds, that all projection images are used for the reconstruction of each section image and that the chest contains organs with movement like the heart, leads to motion artefacts, relatively common in DCT examination. The likelihood of detection of a pulmonary nodule in DCT depends on size, localisations, and density. As we know, the detection rate increases with nodule size with CR (38). Localisation of pleural and subpleural nodular lesions have been identified as a measurement problem in analysing DCT images. The plausible explanation for the difficulty in distinguishing pleural from subpleural nodule location is the limited depth resolution of DCT. High-density opacities such as skeletal changes can be problematic when reading access tomosynthesis examination. For instance, costochondral calcifications may be misinterpreted as nodules. Also, most pleural plaques are calcified and exhibit high density. Another topic is low-attenuation nodules, especially non-solid nodules that even in retrospect may not visible with DT images due to the inferior contrast resolution compared with CT. Furthermore, lymph nodes in hilar and mediastinal node station may be perceived as pulmonary nodules and vice versa (39).
Conclusions
DCT is an imaging tool with a broad and growing range of clinical applications. DCT improves diagnostic accuracy and confidence in the diagnosis of suspected pulmonary lesions on CR. Its sensitivity is superior to CR but inferior to CT. One of its most promising roles may be to reduce cost and radiation dose. Furthermore, DCT examinations can discover another pathological finding not visible on standard CR. DCT could have a wider clinical use shortly if data found in the literature were confirmed by other studies and clinical trials.
In summary, DCT has during its short existence a ready introduced itself a valuable imaging technique, and in future, it may play a significant role in chest radiology.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vallebona A. Axial transverse laminagraphy. Radiology 1950;55:271-3. [Crossref] [PubMed]
- Grant DG. Tomosynthesis: a three-dimensional radiographic imaging technique. IEEE Trans Biomed Eng 1972;19:20-8. [Crossref] [PubMed]
- Dobbins JT 3rd, McAdams HP. Chest tomosynthesis: technical principles and clinical update. Eur J Radiol 2009;72:244-51. [Crossref] [PubMed]
- Dobbins JT 3rd, McAdams HP, Godfrey DJ, et al. Digital tomosynthesis of the chest. J Thorac Imaging 2008;23:86-92. [Crossref] [PubMed]
- Dobbins JT 3rd, Godfrey DJ. Digital x-ray tomosynthesis: current state of the art and clinical potential. Phys Med Biol 2003;48:R65-106. [Crossref] [PubMed]
- Bertolaccini L, Viti A, Terzi A. Digital tomosynthesis in lung cancer: state of the art. Ann Transl Med 2015;3:139. [PubMed]
- Machida H, Yuhara T, Tamura M, et al. Whole-Body Clinical Applications of Digital Tomosynthesis. Radiographics 2016;36:735-50. [Crossref] [PubMed]
- Langer SG, Graner BD, Schueler BA, et al. Sensitivity of Thoracic Digital Tomosynthesis (DTS) for the Identification of Lung Nodules. J Digit Imaging 2016;29:141-7. [Crossref] [PubMed]
- McAdams HP, Samei E, Dobbins J 3rd, et al. Recent advances in chest radiography. Radiology 2006;241:663-83. [Crossref] [PubMed]
- Didkowska J, Wojciechowska U, Manczuk M, et al. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med 2016;4:150. [Crossref] [PubMed]
- Feld R, Sagman U, LeBlanc M. Staging and prognostic factors: Small cell lung cancer. In: Pass HI, Mitchell JB, Johnson DH, et al. editors. Lung Cancer: Principles and Practice. Philadelphia: Lipincott-Raven Publishers, 1996:495-509.
- Nesbitt JC, Lee JS, Komaki R, et al. Cancer of the lung. In: Holland JF, Frei E III, Bast RC Jr, et al. Editors. Cancer Medicine. Fourth edition. Philadelphia: Williams and Wilkins, 1997:1723-803.
- Iribarren C, Tekawa IS, Sidney S, et al. Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men. N Engl J Med 1999;340:1773-80. [Crossref] [PubMed]
- Chou SH, Kicska GA, Pipavath SN, et al. Digital tomosynthesis of the chest: current and emerging applications. Radiographics 2014;34:359-72. [Crossref] [PubMed]
- Bertolaccini L, Viti A, Tavella C, et al. Lung cancer detection with digital chest tomosynthesis: first round results from the SOS observational study. Ann Transl Med 2015;3:67. [PubMed]
- Godfrey DJ, McAdams HP, Dobbins JT 3rd. Optimization of the matrix inversion tomosynthesis (MITS) impulse response and modulation transfer function characteristics for chest imaging. Med Phys 2006;33:655-67. [Crossref] [PubMed]
- Lauritsch G, Haerer W. A theoretical framework for filtered backprojection in tomosynthesis. Proc SPIE 1998;3338:1127-37. [Crossref]
- Badea C, Kolitsi Z, Pallikarakis N. Image quality in extended arc filtered digital tomosynthesis. Acta Radiol 2001;42:244-8. [Crossref] [PubMed]
- Stevens GM, Fahrig R, Pelc NJ. Filtered backprojection for modifying the impulse response of circular tomosynthesis. Med Phys 2001;28:372-80. [Crossref] [PubMed]
- Zhang Y, Chan HP, Sahiner B, et al. A comparative study of limited-angle cone-beam reconstruction methods for breast tomosynthesis. Med Phys 2006;33:3781-95. [Crossref] [PubMed]
- Levakhina YM, Duschka RL, Vogt FM, et al. A dual-axis tilt acquisition geometry for digital musculoskeletal tomosynthesis. Phys Med Biol 2013;58:4827-48. [Crossref] [PubMed]
- Meltzer C, Bath M, Kheddache S, et al. Visibility of Structures of Relevance for Patients with Cystic Fibrosis in Chest Tomosynthesis: Influence of Anatomical Location and Observer Experience. Radiat Prot Dosimetry 2016;169:177-87. [Crossref] [PubMed]
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. [Crossref] [PubMed]
- Bath M, Svalkvist A, von Wrangel A, et al. Effective dose to patients from chest examinations with tomosynthesis. Radiat Prot Dosimetry 2010;139:153-8. [Crossref] [PubMed]
- Yamada Y, Jinzaki M, Hasegawa I, et al. Fast scanning tomosynthesis for the detection of pulmonary nodules: diagnostic performance compared with chest radiography, using multidetector-row computed tomography as the reference. Invest Radiol 2011;46:471-7. [Crossref] [PubMed]
- Silva AC, Lawder HJ, Hara A, et al. Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR Am J Roentgenol 2010;194:191-9. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Grosso M, Priotto R, Ghirardo D, et al. Comparison of digital tomosynthesis and computed tomography for lung nodule detection in SOS screening program. Radiol Med 2017;122:568-74. [Crossref] [PubMed]
- Kim EY, Bista AB, Kim T, et al. The advantage of digital tomosynthesis for pulmonary nodule detection concerning influence of nodule location and size: a phantom study. Clin Radiol 2017;72:796.e1-8. [Crossref] [PubMed]
- Dobbins JT 3rd, McAdams HP, Sabol JM, et al. Multi-Institutional Evaluation of Digital Tomosynthesis, Dual-Energy Radiography, and Conventional Chest Radiography for the Detection and Management of Pulmonary Nodules. Radiology 2017;282:236-50. [Crossref] [PubMed]
- Choo JY, Lee KY, Yu A, et al. A comparison of digital tomosynthesis and chest radiography in evaluating airway lesions using computed tomography as a reference. Eur Radiol 2016;26:3147-54. [Crossref] [PubMed]
- Nemesure B, Plank A, Reagan L, et al. Evaluating efficacy of current lung cancer screening guidelines. J Med Screen 2017. [Epub ahead of print]. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243-53. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Diederich S. Pulmonary nodules: do we need a separate algorithm for non-solid lesions? Cancer Imaging 2009;9 Spec No A:S126-8.
- Lee KH, Goo JM, Lee SM, et al. Digital tomosynthesis for evaluating metastatic lung nodules: nodule visibility, learning curves, and reading times. Korean J Radiol 2015;16:430-9. [Crossref] [PubMed]
- Quaia E, Baratella E, Poillucci G, et al. Diagnostic impact of digital tomosynthesis in oncologic patients with suspected pulmonary lesions on chest radiography. Eur Radiol 2016;26:2837-44. [Crossref] [PubMed]
- Vikgren J, Zachrisson S, Svalkvist A, et al. Comparison of chest tomosynthesis and chest radiography for detection of pulmonary nodules: human observer study of clinical cases. Radiology 2008;249:1034-41. [Crossref] [PubMed]
- Gomi T. X-ray Digital Linear Tomosynthesis Imaging for Artificial Pulmonary Nodule Detection. J Clin Imaging Sci 2011;1:16. [PubMed]


