Emerging uses of biomarkers in lung cancer management
Over the past decade, non-small cell lung cancer (NSCLC) has witnessed unprecedented scientific advances that have translated into meaningful improvements in clinical outcomes. Much of these gains have been predicated on a better understanding of tumor biology including the identification of driver mutations that allow patients to receive novel, genotype-driven therapies. Multiple studies have now demonstrated improvements in outcomes in advanced stage patients EGFR mutations and ALK rearrangements treated with tyrosine kinase inhibitors (TKIs) when compared to chemotherapy. While the precision therapy paradigm was initially restricted to these aforementioned genotypes, a broader genomic characterization of lung cancer via next generation sequencing (NGS) approaches has identified BRAF, MET exon 14 skipping mutations as well as ROS-rearrangements as actionable mutations. Aiding this effort has been the development of novel diagnostic platforms like plasma genotyping (i.e., liquid biopsies) that can identify relevant, genetic alterations in blood when tissue is inadequate. These important diagnostic and therapeutic advances in lung cancer have converged to increase the number of patients eligible for targeted therapies and improved outcomes for these patients.
In addition to targeted therapies, a firmer understanding of tumor immunology and tumor escape mechanisms has led to one of the greatest successes in all of solid tumor oncology—the development of the checkpoint inhibitors, antibodies against programmed death-1 (PD-1) and its ligand (PD-L1). Nivolumab, pembrolizumab, and atezolizumab have dramatically altered the treatment paradigm in NSCLC each demonstrating improved response rates, survival, and importantly, quality of life when compared to chemotherapy. Enrichment strategies to better select those patients more likely to respond have demonstrated PD-L1 staining by immunohistochemistry (IHC) to be a predictive biomarker in both treatment naïve and refractory patients. Further efforts are ongoing to identify other reliable plasma and tissue based biomarkers.
With these remarkable advances come many challenges for the practicing clinician as well as for ongoing research to further improve testing and treatment paradigms. With the ever-increasing numbers of important biomarkers that aid in optimal treatment selection, the need for obtaining adequate tissue and establishing careful algorithms for proper tissue acquisition and stewardship are paramount. The enhanced understanding of molecular mechanisms leading to treatment resistance calls for ongoing molecular and immune monitoring of our patients to be able to guide them towards effective newer generation agents (Figure 1). Dramatic advances in the use of checkpoint inhibitors has been paralleled by the introduction of a range of predictive biomarkers such as PD-L1 testing, assessment of tumor mutation burden and T cell activation signatures and proper utilization of these biomarkers is expected to be clarified in the next few years. Now a new challenge awaits to better define primary and acquired resistance mechanisms against checkpoint blockade calling for repeat biopsies and immune assessments along the continuum of care in our patients more than ever before. The rapid evolution of highly sensitive ctDNA platforms is a key paradigm shift allowing dynamic monitoring in a safe and convenient fashion and further novel platforms are being developed and will be sorely needed for proper guidance on immunotherapeutic agents.

The current special edition of the Annals of Translational Medicine attempts to capture the current state of affairs and anticipated research developments for biomarker identification and utilization for the management of patients with NSCLC. First, West (1) provides an overview of the best evidence on proper usage of upfront molecular biomarkers such as EGFR/ALK/ROS to optimize front-line targeted therapy. Lau et al. (2) expand on this topic to review the rapidly increasing number of validated and emerging actionable targets as well as epigenetic targets calling for expanded molecular profiling in the management of patients with advanced non-squamous NSCLC. Sabari and colleagues (3) discuss findings on recurrent molecular alterations and data on potential targets under investigation for patients with advanced squamous and small cell lung carcinoma. To compliment these manuscripts focusing on molecular targets, Feldman et al. (4) will review the landscape for biomarker testing for earlier stage, curative settings. Switching gears, Matthen et al. (5) will discuss current best evidence and challenges when it comes to the use of PD-L1 testing to guide patient selection towards immunotherapeutic agents and Voong and colleagues (6) expand on novel platforms under development that might find utility to guide checkpoint inhibitor therapy. Next, Attarian et al. (7) provide a review on the current knowledge when it comes to acquired resistance to molecularly targeted and immunotherapeutic agents as well as proper integration of serial testing into our day-to-day management pathways. The last section focuses on the rapid introduction of blood-based biomarker testing into routine practice with Velcheti et al. (8) providing a general review of the available and emerging technology for ctDNA and CTC-based approaches. Next, Singh et al. (9) will review the already carefully validated use of ctDNA testing for upfront EGFR mutation and EGFR T790M detection. Lastly, Marmarelis et al. (10) will explore the large diversity of up and coming opportunities for ctDNA utilization in the clinic to optimize patient care.
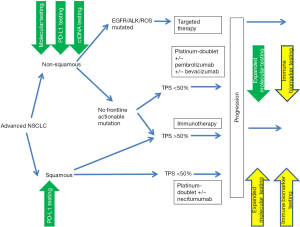
Overall, the last few years have been an extraordinary time in thoracic oncology with unprecedented advances in diagnostics and treatment. Molecular and immune biomarker tests have dramatically expanded our understanding of the molecular genetics, biological behavior, immune and microenvironmental interactions and treatment sensitivities of lung cancers and are part of established practice along the diagnostic and treatment continuum as illustrated in Figure 2. Our hope is that the manuscripts brought forth in this edition will appropriately capture this enthusiasm and provide an overview of and impetus for further investment in biomarker research in the coming years to achieve the best outcomes for our patients.
Acknowledgements
None.
References
- West H. Genotyping for EGFR/ALK/ROS to guide front-line management of non-squamous non-small cell lung cancer. Ann Transl Med 2017;5:371.
- Lau KW, Seng C, Lim TKH, et al. Expanded molecular interrogation for potential actionable targets in non-squamous non-small cell lung cancer. Ann Transl Med 2017;5:372.
- Sabari J, Paik PK. Relevance of genetic alterations in squamous cell and small cell lung cancer. Ann Transl Med 2017;5:373.
- Feldman R, Kim ES. Prognostic and predictive biomarkers post curative intent therapy. Ann Transl Med 2017;5:374.
- Matthen M, Safyan RA, Shu CA. PD-L1 as a biomarker in NSCLC: challenges and future directions. Ann Transl Med 2017;5:375.
- Voong KR, Feliciano J, Becker D, et al. Beyond PDL1 testing. Emerging biomarkers for immunotherapy in non-small cell lung cancer. Ann Transl Med 2017;5:376.
- Attarian S, Rahman N, Halmos B. Molecular testing for acquired drug resistance. Ann Transl Med 2017;5:377.
- Velcheti V, Pennell NA. Non-Invasive Diagnostic Platforms in Management of Non-Small Cell Lung Cancer (NSCLC): Opportunities and Challenges. Ann Transl Med 2017;5:378.
- Singh AP, Li S, Cheng H. Circulating DNA in EGFR-mutated lung cancer. Ann Transl Med 2017;5:379.
- Marmarelis M, Thompson JC, Aggarwal C, et al. Emerging uses of circulating tumor DNA in advanced stage non-small cell lung cancer. Ann Transl Med 2017;5:380.


