Circulating DNA in EGFR-mutated lung cancer
Introduction
Lung cancer is the leading cause of cancer related deaths worldwide with an estimated 155,870 deaths in 2017 in the United States alone (1). Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancers and the cornerstone of management for advanced stage disease has traditionally been cytotoxic chemotherapy, albeit with low response rates (20% to 25%) and overall survival (OS) of 10–12 months (2,3). The discovery of driver mutations in the epidermal growth factor receptor (EGFR) gene in a subset of patients with NSCLC and the development of oral tyrosine kinase inhibitors (TKIs) targeting these mutant receptors has remarkably changed the therapeutic landscape in advanced NSCLC. It has led to improved progression free survival as well as quality of life in this patient population (4). The prevalence of sensitizing EGFR mutation ranges from 14% to 38% in patients with NSCLC depending on geographic location and ethnicity (3). These mutations are more commonly present in women, non-smokers and Asian populations. There are several EGFR TKIs now approved including the first-generation gefitinib and erlotinib, second-generation afatinib, and third-generation osimertinib. Therefore, tumor genotyping is of utmost importance and traditionally performed via invasive fine needle aspiration or core biopsies. Liquid biopsy including analysis with circulating tumor DNA (ctDNA) is an emerging platform to complement tissue biopsies, offering a rapid and minimally invasive method for tumor genotyping.
ctDNA
ctDNA consists of short fragments of double stranded DNA shed from tumors and is characterized by unique somatic mutations that are not present in normal cells. It is shed following necrosis or apoptosis during cell turnover and released into circulation either passively or by active egress via exosomes (5). ctDNA is found in patients with all stages of NSCLC, ranging from 50% of stage I cancers to >90% of advanced staged cancers with the prevalence increasing with the stage and number of metastatic sites (6-8). The fraction of ctDNA found in the blood can vary from less than 0.01% to >90% of total circulating cell free DNA depending on the type, tumor burden or stage of the tumor (9).
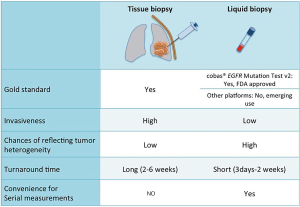
We now have extremely sensitive and specific platforms to detect ctDNA in the blood that can be polymerase chain reaction (PCR) based or next generation sequencing (NGS) based. PCR based assays for example, droplet digital PCR (ddPCR), ARMS and BEAMing are relatively cheap, and highly sensitive and specific but interrogate a limited number of known mutations. NGS-based assays like TAM-Seq and CAPP-Seq, although costly, retain high sensitivity and specificity and are capable of detecting multiple mutations of interest from a single sample (Table 1). PCR based assays cannot easily detect copy number alterations and rearrangements (like ALK or ROS1 fusions), which are more easily detected via NGS (11). These platforms are increasingly being utilized to detect activating EGFR mutations as well as resistance mutations in patients who progress on EGFR TKIs. The U.S. Food and Drug Administration (FDA) approved the real-time PCR based cobas®EGFR Mutation Test v2 to detect the presence of sensitizing EGFR mutations (exon 19 deletion or L858R substitution in exon 21) in peripheral blood to determine which patients are candidates for treatment with erlotinib. It also detects the most common resistance EGFR mutation, T790M to determine which patients will benefit from the third generation TKI, osimertinib.
The ctDNA test is non-invasive, allowing repeated measurements if needed without subjecting patients to the risk of invasive tissue biopsies. Another issue with tissue genotyping is potential poor yield of specimen for extensive molecular testing which may be circumvented by using ctDNA analysis (7,12,13). The ctDNA is also thought to better reflect intra-tumoral heterogeneity since it is released from all parts of the tumor and better replicates the global genomic context of the tumor, compared to tissue biopsies that may miss certain genomic alterations in different tumor sub-clones. The turnaround time of ctDNA is also much faster, yielding results weeks before tissue analysis, providing a more real time assessment of the constantly evolving tumor mutational landscape (Figure 1).
Utilizing ctDNA in initial diagnosis
Clinical benefits from using targeted TKIs in EGFR-mutated lung cancer patients have led the National Comprehensive Care Network (NCCN) to recommend testing patients with advanced non-squamous NSCLC and selected squamous cell carcinoma (never smokers, small biopsy specimens or mixed histology) for EGFR mutations and ALK/ROS1 rearrangements, preferably as part of broader molecular profiling for treatment planning (14,15). Numerous studies have demonstrated that ctDNA is a reliable surrogate for tumor tissue to detect sensitizing EGFR mutations (Table 2).

Full table
A preplanned, exploratory analysis of the phase IV single arm study of gefitinib in EGFR positive NSCLC patients, determined a mutation status concordance rate between 652 matched tumor and plasma samples to be 94.3% with a test sensitivity of 65.7% and specificity of 99.8% using a Scorpion ARMS-based EGFR mutation detection kit (25). A prospective study of 102 patients with advanced NSCLC revealed a concordance between matched plasma and tissue samples for EGFR mutations to be 79% using an NGS based assay (7). The concordance rate improved to 100% as the time between the collections of the two samples was closer to each other. A meta-analysis of 20 studies assessing the performance of ctDNA compared to tissue to detect EGFR mutations in NSCLC, demonstrated a pooled sensitivity of 67% and a pooled specificity of 93% (29). Another meta-analysis of 25 studies demonstrated the pooled overall sensitivity, specificity, and concordance rate of ctDNA compared to tissue as 61%, 90% and 79%, respectively (30). Qiu et al. showed a pooled sensitivity and specificity of 62% and 95.9% for ctDNA to detect EGFR mutations in their meta-analysis of 27 studies involving 3,110 participants (31). The large multicenter non-interventional ASSESS study investigated the utility of ctDNA EGFR testing in the real world setting in patients with metastatic NSCLC in Europe and Japan. The concordance of mutation status in 1,162 matched blood and tissue samples was 89% with a sensitivity of 46%, specificity of 97% and positive predictive value of 78% (19). This data should be interpreted with caution since various different testing platforms were used, as this was not a strictly controlled study but rather a reflection of practice in the real world.
In addition to high concordant rates as mentioned above, several studies have shown ctDNA to be a reliable predictive and prognostic marker in EGFR mutant NSCLC. Kimura et al. in their study of 42 Japanese patients with NSCLC showed that EGFR mutations detected using ctDNA via Scorpion ARMS technology predicted for an objective response to gefitinib as well as improved progression free survival (PFS) (174 vs. 58 days in serum samples), similar to mutations detected in tumor samples (32). Bai et al. showed similar results with improved PFS in advanced NSCLC patients with EGFR mutations detected in plasma, treated with gefitinib compared to those without these mutations (median PFS, 11.1 vs. 5.9 months) (28). In another study, ctDNA in both plasma and pleural effusions was shown to predict PFS and OS in patients treated with gefitinib (33). In the aforementioned gefitinib study by Douillard et al., the overall response rates and progression free survival in NSCLC patients receiving the drug were similar, regardless the EGFR mutation was detected in the tumor or plasma (70% vs. 76.9% and 9.7 vs. 10.2 months, respectively) (25). Several other studies have corroborated these findings establishing a clear predictive role of ctDNA EGFR mutations in the response to TKIs in NSCLC (34-36). A pre-specified analysis of the EURTAC trial comparing first line erlotinib and chemotherapy in advanced NSCLC, assessed ctDNA as a surrogate for EGFR mutation testing (37). Median OS was found to be shorter in patients with the L858R mutation in ctDNA than in those with the exon 19 deletion (13.7 vs. 30.0 months) and patients with L858R mutations detected in both ctDNA as well as tumor had shorter survival compared to patients with the mutation detected only in tumor tissue (13.7 vs. 27.7 months). The mean level of circulating free DNA (cfDNA) has also been shown to predict OS in NSCLC patients, with higher levels correlating with worse survival (38). Levels >3 ng/µL were associated with a median OS of 24 vs. 46 months in one prospective study (7).
The promise of ctDNA is not to entirely replace tissue-base testing, since histology remains as a key determinant to make treatment decisions. However, it does offer a reliable complement to tissue testing particularly in the clinical scenario of suboptimal tissue obtained by biopsies or in patients where repeat biopsies are too risky to perform.
Role of ctDNA in the resistance setting
Despite the excellent response to first generation EGFR TKIs, most patients eventually develop resistance usually within one year of therapy, with the most common resistance mutation being T790M (5). Identifying the resistance mutations is extremely clinically pertinent since the third generation EGFR TKIs like osimertinib have been shown to have high response rates (71% vs. 31%) and prolong PFS compared to chemotherapy (median PFS 10.1 vs. 4.4 months) in EGFR mutated patients who have progressed on prior EGFR TKIs with the development of T790M (39). Numerous studies have yielded evidence that ctDNA can be used to complement tumor DNA testing to detect these genetic alterations. Thress et al. demonstrated the efficacy of two different platforms to detect T790M in blood, with a sensitivity of 73% and 81% and specificity of 67% and 58% (40). In one study, similar objective response rates (ORR) were seen in patients treated with rociletinib, regardless of whether T790M was detected in tumor or plasma (21). Plasma EGFR T790M status was shown to be associated with worse OS in another study (20). A retrospective analysis of patients with acquired resistance to EGFR TKIs revealed that the clinical outcomes in terms of PFS and ORR were similar in patients with T790M positive plasma (ORR 63%; PFS 9.7 months) or tumor (ORR 62%; PFS 9.7 months) (41). The study also concluded that given the 30% false negative rate of plasma T790M testing, those with negative plasma T790M still needed a tissue biopsy to accurately determine the T790M status. Another study assessed the efficacy of osimertinib when T790M status was determined in ctDNA in 48 EGFR-mutant NSCLC patients with disease progression (42). The T790M mutation was detected in 50% of patients, with osimertinib achieving a partial response rate of 62.5% and a stable disease rate of 37.5%. Median PFS was not achieved at the 8-month follow-up. The study concluded that ctDNA is a reliable surrogate for T790M mutation status.
Third generation EGFR TKIs act by covalently binding to the cysteine residue at position 797 in the ATP binding cleft of the EGFR receptor. Despite impressive initial responses, patients eventually develop resistance to these drugs as well, with the acquisition of novel mutations, one of the most common ones being the base substitution, C797S which prevents their binding to the EGFR receptor (43). The context of developing the C797S mutations in relation to other EGFR alleles determines the response to subsequent therapies (44). If the mutation develops in trans-, cells will be sensitive to a combination of first- and third-generation TKIs, whereas mutations in cis- will not respond to EGFR TKIs, alone or in combination. C797S acquired in cells with de novo T790M (when third-generation TKIs are administered in the first-line setting), are resistant to third-generation TKIs but susceptible to first-generation TKIs. Chabon et al. demonstrated that this allelic assessment is possible with the short fragments of ctDNA (~180 bp) likely due to the proximity of the two mutations (45), however the same may be difficult to assess if the mutations of interest are far apart.
Therefore, the clinical utility of ctDNA in the resistance setting is to avoid invasive repeat biopsies in patients with progression of disease. Plasma T790M detection can be relied upon as a true positive and these patients may be able to avoid biopsies. However, if the patient is plasma T790M negative, they still require a tissue biopsy to confirm the results since this may be a false negative and the patient may still benefit from osimertinib. The false negatives of ctDNA testing are believed to be due to non-shedding tumors rather than a fallibility of the testing platforms themselves. In the event that plasma EGFR mutation is positive however tissue mutation is negative, it is often considered that this “false positive” is actually a false negative of tissue testing given the high positive predictive value of ctDNA and may be due to the inability of tissue testing to capture tumoral heterogeneity.
ctDNA in other body fluids
Urine and CSF have also been utilized to assess both sensitizing and resistance EGFR mutations. Reckamp et al. used NGS based assays to interrogate EGFR activating mutations and T790M resistance mutation in urine or plasma of patients enrolled in the TIGER-X trial, a phase 1/2 study of rociletinib in previously treated patients with EGFR positive advanced NSCLC (46). The sensitivity of detecting EGFR mutations in urine was 80% for L858R, 83% for exon 19 deletions and 93% for T790M with a comparable sensitivity observed in plasma. Twelve additional T790M positive patients were identified with both urine and plasma, which were undetectable or inadequate by tissue genotyping. A rapid decrease in T790M levels was noted by day 21 on serial monitoring nine patients while receiving rociletinib. A prospective study evaluated 150 treatment naïve patients with advanced NSCLC positive for EGFR mutations L858R or L861Q and being treated with first generation TKIs (17). Patients were monitored with urine and plasma samples collected every month for 9 months. The overall concordance was 88% between urinary and matched tumor samples and 98% between urinary and plasma cells. Immediately after beginning treatment with TKI, a decline in urinary cfDNA level was observed. However, later during the monitoring period, an increase in cfDNA level and greater variation in cfDNA was noted. 53% of patient developed T790M mutations and these patients had higher urinary levels of cfDNA as well as a worse OS compared to T790M negative patients. Husain et al. demonstrated 100% concordance of urine T790M positivity compared to tissue (47). A biomarker study of EGFR mutant NSCLC patients on EGFR TKIs monitored urine cfDNA prior to and after progression on TKIs (47). An interim analysis showed that urine T790M was detected in 68% of patients (n=15/22 patients) on EGFR TKIs with high concordance. Urine EGFR T790M was detected up to 3 months prior to radiographic progression and urine ctDNA peaked one day after therapy, which predicted radiographic response.
EGFR mutations have been detected in the CSF of patients with leptomeningeal disease (48). A retrospective study used ARMS-PCR assays to interrogate EGFR mutations in 30 lung adenocarcinoma patients with brain metastases (49). Sixteen patients were positive for activating EGFR mutations and the sensitivity of CSF for EGFR mutations was 67% with a specificity of 82%. Another study retrospectively analyzed the CSF of seven EGFR mutant NSCLC patients who had developed leptomeningeal disease during or after therapy with gefitinib (50). The EGFR mutation detected in all cases was the same as that detected in the primary tumor whereas cytology was positive in only two patients. Erlotinib was efficacious in all cases however, eventually all patients progressed. Zhao et al. collected paired CSF and plasma samples from seven NSCLC patients with CNS metastases after EGFR TKI failure (51). EGFR mutations were detected in all seven CSF samples, while 5 of the 7 matched plasma samples were negative for EGFR mutations. After TKI failure, majority of the patients with CNS metastases remained positive for EGFR sensitive mutations in CSF, but much less in the matched plasma. Another study concluded that CSF ctDNA better captures genetic alterations in patients with brain metastases compared to plasma ctDNA (52).
EGFR mutations in NSCLC patients have also been detected in saliva using electric field-induced release and measurement (EFIRM) with receiver operating characteristic analysis indicating that EFIRM detected exon 19 deletion with an area under the curve (AUC) of 0.94 and the L858R mutation with an AUC of 0.96 (53). However, this platform is not in widespread use clinically for the time being.
Park et al. isolated cfDNA from bronchoalveolar lavage (BAL) fluid and bronchial washing samples from 20 lung adenocarcinoma patients and interrogated them for EGFR mutations using peptide nucleic acid (PNA)-mediated PCR clamping method (54). In cases where tumor biopsy and cfDNA test were not concordant, PANAMutyper™ R EGFR kit was utilized in addition. The results from PNA-mediated PCR clamping were 75% (n=9/12) concordant with tumor EGFR mutation status. PANAMutyper with fluorescence melting curve analysis was performed in three cases, which detected EGFR mutations in two more patients (n=11/12, 91.7%).
Role in longitudinal clinical monitoring
The non-invasive nature of ctDNA as well as quick turnaround time for results lends it to being a promising test for serial monitoring of patients on treatment. Marchetti et al. performed serial EGFR testing at baseline and then 4 to 60 days during TKI therapy in EGFR mutant NSCLC patients (55). They noted that the level of ctDNA correlated with tumor shrinkage. There was a more than 50% reduction of EGFR copy number at 14 days in rapid responders compared to patients that were slow responders. A prospective analysis of advanced NSCLC patients demonstrated that patients with complete resolution of ctDNA at either 2 or 6 weeks after treatment, exhibited a lower treatment discontinuation rate (0% at initial and 4% at second reimaging) compared to patients without complete resolution (33% at initial and 56% at second reimaging), presumably correlating with radiographic response and emergence of acquired resistance (11). Another prospective study followed 62 EGFR mutant NSCLC patients receiving TKI therapy with serial plasma ctDNA testing (56). Failure to clear plasma EGFR mutations after TKI therapy predicted for worse PFS and OS. An exploratory analysis comparing matched tumor and blood samples from the FASTACT-2 study assessed the clinical outcome of NSCLC patients treated with first line chemotherapy with erlotinib (36). For plasma EGFR mutant patients, median PFS (7.2 vs. 12.0 months) and OS (18.2 vs. 31.9 months) were shorter for patients who remained plasma EGFR positive by cycle 3 compared to those who became negative. Lee et al. analyzed EGFR exon 19 deletion, L858R and T790M using ddPCR in 367 serial plasma samples from 81 NSCLC patients treated with EGFR TKIs (57). All 40 patients with EGFR mutations at baseline showed a significant reduction of mutant copies (>50%) in plasma during the first 2 months after treatment. Median PFS was demonstrated to be longer in patients with undetectable EGFR after 2 months compared to those with detectable EGFR mutations (10.1 vs. 6.3 months). A pre-planned exploratory analysis of a randomized phase III trial comparing erlotinib with gefitinib in advanced EGFR mutated NSCLC patients aimed to measure changes in plasma EGFR L858R mutation during EGFR-TKI treatment (58). Serial plasma L858R were detected using quantitative PCR in 80 patients, with a decrease in quantity of L858R to its lowest level noted at the time of best response to EGFR-TKI. Two dynamic types of L858R were found—ascend type and stable type, depending on the level found in patients at disease progression. Median PFS and OS were longer in the ascend types compared to stable types (median PFS, 11.1 vs. 7.5 months; median OS, 19.7 vs. 16.0 months.
Conclusions
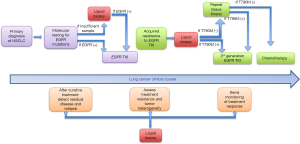
We propose the following model to utilize ctDNA in the context of EGFR mutant NSCLC (Figure 2). For initial diagnosis, tissue biopsy remains the gold standard since identifying histology (e.g., small cell vs. non-small cell) remains extremely relevant. If adequate tissue is not available, we recommend obtaining ctDNA to look for driver EGFR mutations, which have important predictive and prognostic properties. At the time of progression of disease while on first generation EGFR TKIs, we recommend sending ctDNA testing to interrogate for resistance mutations like T790M. If positive, one can proceed with using a third generation TKI given the extremely high positive predictive value using newer testing platforms. If ctDNA is negative for T790M, repeat tissue biopsy is still recommended due to the 30% false negative rate of ctDNA.
In conclusion, ctDNA is an exciting and promising technology, which is fast becoming an attractive complement to tissue-based genotyping for initial diagnosis, and detection of resistance at disease progression in EGFR mutated NSCLC. With future research, emerging uses in disease surveillance and detection of minimal residual disease will continue to harness the role of ctDNA in the era of precision medicine.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society: Cancer Facts and Figures 2017. Atlanta, Ga: American Cancer Society, 2017. Available online: https://old.cancer.org/acs/groups/content/@editorial/documents/document/acspc-048738.pdf
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 2016;7:78985-93. [PubMed]
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595-605. [Crossref] [PubMed]
- Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 2014;32:579-86. [Crossref] [PubMed]
- Chen KZ, Lou F, Yang F, et al. Circulating Tumor DNA Detection in Early-Stage Non-Small Cell Lung Cancer Patients by Targeted Sequencing. Sci Rep 2016;6:31985. [Crossref] [PubMed]
- Thompson JC, Yee SS, Troxel AB, et al. Detection of Therapeutically Targetable Driver and Resistance Mutations in Lung Cancer Patients by Next-Generation Sequencing of Cell-Free Circulating Tumor DNA. Clin Cancer Res 2016;22:5772-82. [Crossref] [PubMed]
- Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med 2014;20:548-54. [Crossref] [PubMed]
- Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008;14:985-90. [Crossref] [PubMed]
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017;14:531-48. [Crossref] [PubMed]
- Sacher AG, Paweletz C, Dahlberg SE, et al. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol 2016;2:1014-22. [Crossref] [PubMed]
- Lanman RB, Mortimer SA, Zill OA, et al. Analytical and Clinical Validation of a Digital Sequencing Panel for Quantitative, Highly Accurate Evaluation of Cell-Free Circulating Tumor DNA. PLoS One 2015;10:e0140712. [Crossref] [PubMed]
- Sueoka-Aragane N, Katakami N, Satouchi M, et al. Monitoring EGFR T790M with plasma DNA from lung cancer patients in a prospective observational study. Cancer Sci 2016;107:162-7. [Crossref] [PubMed]
- (National Comprehensive Cancer Network) NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer 2017 (Version 3.2017; November 16, 2016). Availabl online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Lin JJ, Cardarella S, Lydon CA, et al. Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J Thorac Oncol 2016;11:556-65. [Crossref] [PubMed]
- Jenkins S, Yang JC, Ramalingam SS, et al. Plasma ctDNA Analysis for Detection of the EGFR T790M Mutation in Patients with Advanced non-small Cell Lung Cancer. J Thorac Oncol 2017;12:1061-70. [Crossref] [PubMed]
- Chen S, Zhao J, Cui L, et al. Urinary circulating DNA detection for dynamic tracking of EGFR mutations for NSCLC patients treated with EGFR-TKIs. Clin Transl Oncol 2017;19:332-40. [Crossref] [PubMed]
- Wu YL, Sequist LV, Hu CP, et al. EGFR mutation detection in circulating cell-free DNA of lung adenocarcinoma patients: analysis of LUX-Lung 3 and 6. Br J Cancer 2017;116:175-85. [Crossref] [PubMed]
- Reck M, Hagiwara K, Han B, et al. ctDNA Determination of EGFR Mutation Status in European and Japanese Patients with Advanced NSCLC: The ASSESS Study. J Thorac Oncol 2016;11:1682-9. [Crossref] [PubMed]
- Zheng D, Ye X, Zhang MZ, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep 2016;6:20913. [Crossref] [PubMed]
- Sequist LV, Goldman JW; Wakelee HA, et al. Efficacy of rociletinib (CO-1686) in plasma-genotyped T790M-positive non-small cell lung cancer (NSCLC) patients (pts). J Clin Oncol 2015;33;abstr 8001.
- Weber B, Meldgaard P, Hager H, et al. Detection of EGFR mutations in plasma and biopsies from non-small cell lung cancer patients by allele-specific PCR assays. BMC Cancer 2014;14:294. [Crossref] [PubMed]
- Jing CW, Wang Z, Cao HX, et al. High resolution melting analysis for epidermal growth factor receptor mutations in formalin-fixed paraffin-embedded tissue and plasma free DNA from non-small cell lung cancer patients. Asian Pac J Cancer Prev 2014;14:6619-23. [Crossref] [PubMed]
- Wang S, Han X, Hu X, et al. Clinical significance of pretreatment plasma biomarkers in advanced non-small cell lung cancer patients. Clin Chim Acta 2014;430:63-70. [Crossref] [PubMed]
- Douillard JY, Ostoros G, Cobo M, et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol 2014;9:1345-53. [Crossref] [PubMed]
- Zhao X, Han RB, Zhao J, et al. Comparison of epidermal growth factor receptor mutation statuses in tissue and plasma in stage I-IV non-small cell lung cancer patients. Respiration. 2013;85:119-25. [Crossref] [PubMed]
- Huang Z, Wang Z, Bai H, et al. The detection of EGFR mutation status in plasma is reproducible and can dynamically predict the efficacy of EGFR-TKI. Thorac Cancer 2012;3:334-40. [Crossref]
- Bai H, Mao L, Wang HS, et al. Epidermal growth factor receptor mutations in plasma DNA samples predict tumor response in Chinese patients with stages IIIB to IV non-small-cell lung cancer. J Clin Oncol 2009;27:2653-9. [Crossref] [PubMed]
- Luo J, Shen L, Zheng D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis. Sci Rep 2014;4:6269. [Crossref] [PubMed]
- Mao C, Yuan JQ, Yang ZY, et al. Blood as a Substitute for Tumor Tissue in Detecting EGFR Mutations for Guiding EGFR TKIs Treatment of Nonsmall Cell Lung Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94:e775. [Crossref] [PubMed]
- Qiu M, Wang J, Xu Y, et al. Circulating tumor DNA is effective for the detection of EGFR mutation in non-small cell lung cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2015;24:206-12. [Crossref] [PubMed]
- Kimura H, Suminoe M, Kasahara K, et al. Evaluation of epidermal growth factor receptor mutation status in serum DNA as a predictor of response to gefitinib (IRESSA). Br J Cancer 2007;97:778-84. [Crossref] [PubMed]
- Jian G, Songwen Z, Ling Z, et al. Prediction of epidermal growth factor receptor mutations in the plasma/pleural effusion to efficacy of gefitinib treatment in advanced non-small cell lung cancer. J Cancer Res Clin Oncol 2010;136:1341-7. [Crossref] [PubMed]
- Brevet M, Johnson ML, Azzoli CG, et al. Detection of EGFR mutations in plasma DNA from lung cancer patients by mass spectrometry genotyping is predictive of tumor EGFR status and response to EGFR inhibitors. Lung Cancer 2011;73:96-102. [Crossref] [PubMed]
- Goto K, Ichinose Y, Ohe Y, et al. Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol 2012;7:115-21. [Crossref] [PubMed]
- Mok T, Wu YL, Lee JS, et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin Cancer Res 2015;21:3196-203. [Crossref] [PubMed]
- Karachaliou N, Mayo-de las Casas C, Queralt C, et al. Association of EGFR L858R Mutation in Circulating Free DNA With Survival in the EURTAC Trial. JAMA Oncol 2015;1:149-57. [Crossref] [PubMed]
- Ai B, Liu H, Huang Y, et al. Circulating cell-free DNA as a prognostic and predictive biomarker in non-small cell lung cancer. Oncotarget 2016;7:44583-95. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015;90:509-15. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Remon J, Caramella C, Jovelet C, et al. Osimertinib benefit in EGFR-mutant NSCLC patients with T790M-mutation detected by circulating tumour DNA. Ann Oncol 2017;28:784-90. [PubMed]
- Yu HA, Tian SK, Drilon AE, et al. Acquired Resistance of EGFR-Mutant Lung Cancer to a T790M-Specific EGFR Inhibitor: Emergence of a Third Mutation (C797S) in the EGFR Tyrosine Kinase Domain. JAMA Oncol 2015;1:982-4. [Crossref] [PubMed]
- Niederst MJ, Hu H, Mulvey HE, et al. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin Cancer Res 2015;21:3924-33. [Crossref] [PubMed]
- Chabon JJ, Simmons AD, Lovejoy AF, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun 2016;7:11815. [Crossref] [PubMed]
- Reckamp KL, Melnikova VO, Karlovich C, et al. A Highly Sensitive and Quantitative Test Platform for Detection of NSCLC EGFR Mutations in Urine and Plasma. J Thorac Oncol 2016;11:1690-700. [Crossref] [PubMed]
- Husain H, Kosco K, Guerrero S, et al. Detection of EGFR T790M mutation in urinary circulating tumor DNA from metastatic non-small cell lung cancer patients. Ann Oncol 2015;26:i10-4. [Crossref]
- Shingyoji M, Kageyama H, Sakaida T, et al. Detection of epithelial growth factor receptor mutations in cerebrospinal fluid from patients with lung adenocarcinoma suspected of neoplastic meningitis. J Thorac Oncol 2011;6:1215-20. [Crossref] [PubMed]
- Yang H, Cai L, Zhang Y, et al. Sensitive detection of EGFR mutations in cerebrospinal fluid from lung adenocarcinoma patients with brain metastases. J Mol Diagn 2014;16:558-63. [Crossref] [PubMed]
- Sasaki S, Yoshioka Y, Ko R, et al. Diagnostic significance of cerebrospinal fluid EGFR mutation analysis for leptomeningeal metastasis in non-small-cell lung cancer patients harboring an active EGFR mutation following gefitinib therapy failure. Respir Investig 2016;54:14-9. [Crossref] [PubMed]
- Zhao J, Ye X, Xu Y, et al. EGFR mutation status of paired cerebrospinal fluid and plasma samples in EGFR mutant non-small cell lung cancer with leptomeningeal metastases. Cancer Chemother Pharmacol 2016;78:1305-10. [Crossref] [PubMed]
- De Mattos-Arruda L, Mayor R, Ng CK, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 2015;6:8839. [Crossref] [PubMed]
- Wei F, Lin CC, Joon A, et al. Noninvasive saliva-based EGFR gene mutation detection in patients with lung cancer. Am J Respir Crit Care Med 2014;190:1117-26. [Crossref] [PubMed]
- Park S, Hur JY, Lee KY, et al. Assessment of EGFR mutation status using cell-free DNA from bronchoalveolar lavage fluid. Clin Chem Lab Med 2017;55:1489-95. [Crossref] [PubMed]
- Marchetti A, Palma JF, Felicioni L, et al. Early Prediction of Response to Tyrosine Kinase Inhibitors by Quantification of EGFR Mutations in Plasma of NSCLC Patients. J Thorac Oncol 2015;10:1437-43. [Crossref] [PubMed]
- Tseng JS, Yang TY, Tsai CR, et al. Dynamic plasma EGFR mutation status as a predictor of EGFR-TKI efficacy in patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol 2015;10:603-10. [Crossref] [PubMed]
- Lee JY, Qing X, Xiumin W, et al. Longitudinal monitoring of EGFR mutations in plasma predicts outcomes of NSCLC patients treated with EGFR TKIs: Korean Lung Cancer Consortium (KLCC-12-02). Oncotarget 2016;7:6984-93. [Crossref] [PubMed]
- Zhou Q, Yang JJ, Chen ZH, et al. Serial cfDNA assessment of response and resistance to EGFR-TKI for patients with EGFR-L858R mutant lung cancer from a prospective clinical trial. J Hematol Oncol 2016;9:86. [Crossref] [PubMed]