Sedation and neuromuscular blocking agents in acute respiratory distress syndrome
Introduction
Acute respiratory distress syndrome (ARDS) is characterized by non-cardiogenic inflammatory lung oedema with severe hypoxemia. ARDS mortality remains high in 35–40% of patients (1). Invasive mechanical ventilation (MV) is the main organ replacement therapy in patients presenting with ARDS. A lung protective ventilation including reduction of tidal volume at 6 mL/kg predicted body weight and with a limitation of plateau pressure has improved the prognosis of ARDS patients. Sedation is necessary to initiate MV. The aim of this sedation is to provide better adaptation to the ventilator during controlled ventilation and thereby allow a reduction of the tidal volume and limit the plateau pressure under 28–30 cmH2O. During moderate to severe ARDS, deep sedation allows the use of a prone position. However, to fulfil the rules of lung-protective MV and allow the prone position, deep sedation is frequently unavoidable. In some cases, sedation alone is often insufficient to inhibit the central respiratory drive.
Neuromuscular blocking agents (NMBAs) are part of the pharmacological arsenal used in ARDS. However, given the risk for muscular paresis, especially when corticosteroids are used, the use of NMBAs has remained controversial and lacking a clear recommendation until recently. However, the ACURASYS study (2) showed a benefit of the early use of a 48-hour course of NMBAs on the outcomes of ARDS patients. This latter study has renewed the debate on the use of NMBAs in ARDS treatment. The purpose of this review is to summarize the literature on the benefits and adverse effects of the use of sedation and NMBAs in ARDS patients.
Sedation in ARDS patients
Sedation and analgesia: pros and cons in ARDS patients
Sedation is the most frequent prescription in invasively ventilated patients. It frequently associated with anxiolytic (benzodiazepine and propofol) and opiate use. It is undeniable that pain occurs in invasive mechanically ventilated patients. Sedation is mandatory to facilitate tolerance of the intubation tube, endotracheal suction and prolonged immobility in bed (3-5). In ARDS patients in particular, sedation has been used to maintain protective MV. Sedation allows better adaptation, reduces patient-ventilator asynchronies, and prevents the patient from “fighting” the ventilator (6). Sedation also reduces oxygen consumption by reducing spontaneous muscular activity during hypoxemia (7). During severe to moderate ARDS, several therapeutic techniques require deep sedation, such as the prone position, extra-corporal membrane oxygenation and high frequency oscillation ventilation.
The role of sedation to facilitate the patient comfort during care and nursing is unquestionable. Nevertheless, the deleterious effects of sedation in the intensive care unit (ICU) are well demonstrated, although no trial focusing on the ARDS population is available. Many studies on daily strategies for sedation have shown their positive impact on the prognosis of ventilated patients. In a controlled, randomized study of 128 patients, Kress et al. (8) reported a shorter MV time and a reduction in the duration of hospitalization in the ICU during daily group sedation interruption without increasing the adverse effects. Another study reported a reduction in infectious complications, particularly a reduction in ventilated-acquired pneumonia (VAP) in the group receiving a lower dose of sedation (9). However, the respective roles of decreasing sedation and SV in MV are difficult to distinguish. Furthermore, most patients included did not present with ARDS, and all severe cases of ARDS were excluded from these studies. Furthermore, the prone position was not used in these studies.
Moreover, the long-term effects of sedation in ICU patients are still debated. The quality of life is significantly impaired among survivors of critical illness (10,11). Frequently, patients have symptoms of depression, anxiety and post-traumatic stress disorder (PTSD) (12-14). In ICU survivors, the prevalence of post-ICU PTSD is 10–39% at hospital discharge (14). In survivors of ARDS, a systematic review found a prevalence of psychiatrically diagnosed PTSD in 44% at hospital discharge (15). High levels of agitation and delirium in the ICU have been identified as predictors of PTSD (16). This suggests the use of physical restraints is associated with a greater risk of PTSD (17). In addition to the use of physical restraints, sedative medications are often administered to control agitation and anxiety. Benzodiazepine administration is involved in the occurrence of ICU delirium (18) and, consequently, in the occurrence of PTSD. Both midazolam infusion (19,20) and total posology if lorazepam is administered in the ICU are associated with a higher risk of PTSD. High levels of morphine are associated with an increased risk of post-ICU PTSD (21). Minimization of sedation (22), daily interruption (23) of sedation and analgesia based on sedation protocol (24) are strategies associated with a reduction of post-ICU PTSD syndrome.
These data call for a sedation monitoring to limit the deleterious effects of sedatives while ensuring patient comfort and allowing technical procedures, such as the prone position. The use of sedation and analgesia scores represents a basic approach. The Richmond agitation-sedation scale (RASS) and sedation agitation scale (SAS) are the most valid and reliable assessment tools for measuring the quality and depth of sedation in adult ICU patients. These scores are hetero-evaluations processed by ICU nurses. They are recommended by the international guidelines on sedation in adult ICU patients (25). Sedation monitoring is also possible in the ICU, with the bi-spectral index of the electroencephalogram (BIS). There is an overall correlation between BIS monitoring and the clinical sedation scale (26). This is a non-invasive, reproducible and inexpensive monitoring technique (27). The observed BIS value may be increased by muscular contractions, which are abolished by NMBAs. The objective testing of sedation for ARDS patients receiving NMBAs by BIS can be used to quantify the depth of sedation. This monitoring prevents awareness with recall and may reduce the drug requirements. The appropriate BIS range seems to be inferior to 60 (28,29), but in some patients, high BIS values are observed at the deep sedation level (30). Despite the advantages of this technique, systematic monitoring is not recommended in the ICU. Nevertheless, a recent systematic review suggests that a powered, randomized, controlled study is needed for this routine monitoring modality in ventilated patients (31). Ensuring sufficient sedation before paralysis with NMBAs is very important. For ethical reasons, an infusion of NMBAs is inconceivable without sedation. In a prospective, multicentre study in a medical ICU, NMBAs were correctly identified as non-analgesic and non-anxiolytic by 96% of prescribers (32). As described in anaesthesia, awareness with recall results from the absence of or incomplete sedation with NMBAs (33). This situation generates paralysis with a preserved conscience and exposes the patient to psychological trauma (16) and medico-legal procedures (34). In the ACURASYS study (2), all patients were deeply sedated with a Ramsay score at 6 before receiving NMBAs to avoid this adverse effect.
Sedation and analgesia drugs for ARDS patients
Sedation in mechanically ventilated patients is not specific for ARDS. Surveys of clinical practice have reported that most sedation in ventilated patients is achieved with opioids and benzodiazepines (35,36). The most commonly used benzodiazepines are midazolam and lorazepam, and continuous infusion is a more common modality than repeated intravenous boluses. Sufentanyl is the most commonly prescribed opioid in continuous infusion based on two studies of French practices (35,36). Clinical practice guidelines for managing sedation in ICU recommended this association (25).
The type of sedation could have a specific effect on ARDS. In a recent randomized controlled pilot study, Jabaudon et al. (37) evaluated the effects of early sedation with inhaled sevoflurane on arterial oxygenation and lung markers of inflammation. Twenty-five patients with moderate ARDS were included. In the inhaled sedation group, the PaO2/FiO2 ratio was significantly increased (205±56 vs. 166±59 mmHg) at day 2 from ventilation compared to the midazolam group. The two groups were comparable at inclusion in terms of haematosis and ventilator parameters, and all patients received NMBA for a train of four (TOF) at 0 response. Moreover, on day 2, the plasma and alveolar markers of epithelial injury levels and pro-inflammatory cytokines were lower in the sevoflurane group than in the midazolam group. The authors concluded that inhaled sevoflurane for early sedation in ARDS is safe and effective. These results are interesting because sevoflurane has muscle-relaxant and anti-inflammatory properties. Its use in association with conventional sedation could increase the effect of muscle relaxation and might be particularly useful in patients requiring high sedation doses. This study suggests that volatile anaesthetic agents can be used in patients with mild ARDS.
Ketamine is an agonist N-methyl-D-aspartate (NMDA) receptor that can be used for adjunctive sedation in patients with sedation difficulty (drug addicts or patients with regular opioid consumption). There is no specific indication for the sedation of ARDS patients who are ventilated.
Dexmedetomidine is a selective alpha-2 receptor agonist with minor respiratory effects. Dexmedetomidine reduces asynchrony of the patient-ventilator in the weaning time (38) and reduces the duration of MV (39). Dexmedetomidine allows titrated sedation, preserving contact and SV (cooperative sedation). No study has investigated its use in the ARDS patient population. This cooperative sedation must be used as second-line management of ARDS, after the initial inflammatory stage, and its objective is to promote spontaneous breathing.
Control the sedation to promote SV in ARDS: yes, but not too early
Decreasing sedation helps to promote SV in the management of ARDS patients. In the 2000s, particularly at the instigation of Putensen et al. (40), SV permitted by bilevel positive airway pressure (BIPAP™) or airway pressure release ventilation (APRV) appeared to be an elegant technique to reduce lung atelectasis and preserve diaphragm inspiratory efforts. Indeed, the potential benefits of SV during ARDS are as follows:
- Hemodynamic amelioration: by reducing sedation posology (40) and the intra-thoracic pressure caused by SV (41);
- Reduction of diaphragmatic dysfunction: MV causes muscular and diaphragm atrophy with initiation (42,43). The preservation of spontaneous breathing allows the persistence of diaphragmatic contractions and ameliorates the recruitment of dependent zones (44). In a prospective study, Yoshida et al. observed a 41% reduction in atelectasis on thorax CT scans when SB was allowed (45);
- Increasing alveolar recruitment: in an experimental animal study, Henzler et al. (46) observed improvement in the inspiratory transpulmonary pressure in SV. In ARDS patients, a redistribution of ventilation to the pulmonary bases is caused by diaphragmatic contractions. In these studies, spontaneous cycles increased oxygenation and reduced the duration of ventilation.
However, if the beneficial effects of SV are attractive, the results of a large RCT published over the last two decades are inconsistent with this hypothesis. First, all studies that showed a benefit in ARDS mortality (2,47-49) used assisted controlled ventilation (ACV). To date, clinical data for using SV in the early management of ARDS are limited. Putensen et al. (40) compared APRV versus pressure-controlled ventilation (PCV) in a small group of 30 patients and included only 5 ARDS patients with a PaO2/FiO2 ratio <200 mmHg. Putensen et al. (40) concluded that maintaining spontaneous breathing during APRV requires less sedation and improves cardiopulmonary function, which presumably occurs by recruiting non-ventilated lung units, thus requiring a shorter duration of ventilatory support and ICU stay. A study that is currently underway, the BiRDS (NCT 01862016) study, is evaluating two ventilatory strategies on the mortality of ARDS ventilated patients in a controlled, prospective, randomized, open trial. Patients are randomized to ACV or a ventilatory mode authorizing spontaneous breathing cycles superimposed on assistance delivered by the ventilator (BIPAP-APRV mode). Nevertheless, in the first 24 h of study, AVC with deep sedation and NMBAs are used to test SV. The results of this study will offer new data on SV in early ARDS. The main clinical data to challenge the concept of a beneficial effect of spontaneous breathing during MV in the early phase of ARDS are given by Papazian et al. In these trials (2,47,50), deep sedation (Ramsay score 6) and profound neuromuscular blockage with high doses of cisatracurium were used for 48 hours during the early phase of ARDS. With this regimen that prevented any spontaneous breathing, Gainnier et al. showed an improvement in oxygenation (50), a decrease in lung inflammation (51) and an improvement in survival (2).
In all studies, the absence of spontaneous breathing during MV is obtained with deep sedation associated with neuromuscular blockage. We chose to present arguments suggesting that SB could be deleterious in the early phase of ARDS in the NMBAs section (infra).
NMBAs in ARDS patients
State of use and outcome effects
The use of NMBAs in the ICU, especially in ARDS patients, is not marginal. A national US survey performed after the 1995 ACCM/SCCM guidelines implementation showed that vecuronium (Organon Pharmaceuticals, NJ, USA) was routinely used in 18.6% and frequently used in 30.5% of ICU patients (52). In Europe, a Danish study observed that NMBAs were used in 20% of ventilated patients (53). In the ARMA study of the NIH ARDS Network (47), 25% of the 902 patients with ARDS were receiving NMBAs on enrolment. Arroliga et al. (54) reported the administration of NMBAs for at least 1 day in 13% of 5,183 ventilated adult patients and in 38% of 231 ARDS patients. More recently, Arroliga et al. (55) showed that in 549 ARDS patients enrolled in the ALVEOLI trial, NMBAs were used in 45% and 33% of the patients included in the lower and higher positive end-expiratory pressure (PEEP) groups, respectively. Nevertheless, several surveys have reported huge variations in the use of neuromuscular blockade (56-58). Frequently, NMBAs are used for a short period of time (approximately 1±2 days) with daily re-evaluation (52,55,57). However, Rhoney et al. (52) showed that the use of NMBAs was prolonged beyond 72 hours in 10% to 20% of ICU patients.
The most common causes cited by ICU physicians for administering NMBAs are hypoxemia, facilitation of MV and control of patient/ventilator asynchrony (58-60). Factors that have been found to be associated with NMBAs use are mainly related to the disease severity, as assessed by a high APACHE III score and lung injury caused by trauma, sepsis and multiple transfusions (55). Moreover, the use of prone positioning (49), permissive hypercapnia, a high PEEP level, extra-corporeal membrane oxygenator (61) or high-frequency oscillatory ventilation may require the use of NMBAs (62). In 2016, the clinical guidelines practice for sustained NMBA use in critically ill patients (56) recommended (Grade 2C) a short course (48 hours) of paralysis for ARDS patients ventilated with a PaO2/FiO2 ratio inferior to 150. In 2017, the surviving sepsis campaign again recommended (Grade 2C) a short course of infusion of NMBA in ARDS with a PaO2/FiO2 ratio lower than 150 mmHg (63). This recommendation for ARDS is based on 3 clinical studies with 413 patients (2,50,51). This strategy has demonstrated a reduction in the mortality in early ARDS (2).
Data that directly examine the impact of NMBAs on oxygenation in ARDS using a RCT design in the era of lung-protective ventilation are, to the best of our knowledge, limited to three studies. In a multicentre, prospective, controlled, and randomized trial on 56 patients with ARDS in four adult ICUs, Gainnier et al. (50) showed a significant beneficial effect on the course of PaO2/FiO2 ratio in the NMBA treated group of patients compared with the control group. Individual comparisons at each time point indicated that after 48 hours of muscle paralysis, patients who were randomized to NMBA had a higher PaO2/FiO2 ratio at 48, 96, and 120 hours after randomization. In contrast, there was no modification of the PaO2/FiO2 ratio at 1 hour after randomization in the NMBA group. In a second multicentre, prospective RCT that was designed to analyse the inflammatory effects of early 48-hour cisatracurium infusion in ARDS patients, the same group (51) confirmed the beneficial effect of NMBAs on oxygenation in 36 ARDS patients. The PaO2/FiO2 ratio was significantly higher in the NMBA group from the 72th hour, and this effect persisted until the 120th hour (end of the study period observation). The decrease in plateau pressure, PEEP and FiO2 requirements during the 120-hour period of the study were more marked in the NMBAs group. In a more recent study designed by the ACURASYS group (2) to investigate the effects of early NMBA use on ARDS mortality, the PaO2/FiO2 ratio was higher on day 7 in patients receiving 48 hours of continuous cisatracurium infusion than in the control group.
Very few studies have evaluated the effects of NMBA administration on the PaCO2 level. Conti et al. (64) found no variation in the PaCO2 after the administration of NMBAs in 13 patients ventilated for exacerbation of chronic obstructive pulmonary disease or trauma (including 4 ARDS). Lagneau et al. (65) observed no change in the PaCO2 after cisatracurium administration. Gainnier et al. (50) and Forel et al. (51) showed that the PaCO2 was not influenced by a 48-hour continuous administration of cisatracurium. In the ACURASYS study (2), the PaCO2 value was lower after 48 hours in the cisatracurium group than in the control group.
The beneficial effects of NMBAs on oxygenation during ARDS observed in these earlier studies inspired the same group (50,51) to perform a RCT designed to assess the effects of NMBAs on moderate to severe ARDS outcomes. In this multicentre (2), double-blind trial, 340 patients presenting with severe ARDS within the previous 48 hours were randomly assigned to receive either cisatracurium besylate (178 patients) or placebo (162 patients) for 48 hours. The main result of this study was an improvement of the adjusted 90-day survival rate in the group of severe ARDS patients treated early with cisatracurium for 48 hours. After adjusting for both the baseline PaO2/FiO2 and plateau pressure and the simplified acute physiology II score, the hazard ratio for death at 90 days in the cisatracurium group, compared with the placebo group, was 0.68 [95% confidence interval (CI), 0.48 to 0.98; P=0.04]. Furthermore, the mortality at 28 days was 23.7% with cisatracurium and 33.3% with placebo (P=0.05). The beneficial effect of cisatracurium on the 90-day survival rate particularly affected patients presenting a PaO2/FiO2 ratio less than 120 mmHg. Among these patients, the 90-day mortality was 30.8% in the cisatracurium group and 44.6% in the control group (P=0.04). Furthermore, the cisatracurium group had significantly more ventilator-free days than the placebo group during the first 28 and 90 days, and more days were free of organ failure (other than the lung) during the first 28 days. The authors also found that significantly more days were spent outside the ICU between days 1 and 90 in the cisatracurium group. These results tend to show a benefit of NMBAs in the 48 first hours of ARDS regarding the morbidity and mortality. These results are currently being re-evaluated by the ROSE study of the PETAL group (NCT 02509078) to confirm the beneficial effect on mortality of systematic early neuromuscular blockade in ARDS.
One of the limits to the use of NMBAs in the ICU is the occurrence of ICU-acquired weakness (ICUAW). The incidence of ICUAW is 34–60% in patients with ARDS (66). The supposed association between ICUAW and NMBAs is often responsible for a distrust of paralytics. However, independent risk factors of ICUAW clearly identified regroup female sex, multiple organ dysfunction (≥2), duration of MV and administration of corticosteroids (67). The duration of vasopressor support, duration of ICU stays, hyperglycaemia, low serum albumin and neurological failure have also been described as risk factors (68). For NMBAs, several studies (69,70) have found that they were not independently associated with muscular weakness. However, three circumstances appeared to favour the development of ICUAW: the concomitant use of NMBAs and corticosteroids, the use of steroid NMBAs and the length of infusion exceeding 48 hours (71,72). Furthermore, in a recent meta-analysis of the RCT evaluating the use of NMBAs in ARDS (73), cisatracurium was not associated with an increased risk of ICUAW. To summarize, there is no evidence that non-steroid NMBAs, when used for a short duration and without the simultaneous administration of corticosteroids, increase the risk of ICUAW. Interestingly, in the ACURASYS study (2), the incidence of ICU-acquired paresis (evaluated on the basis of the MRC score on day 28 or at the time of ICU discharge) was not lower in patients receiving 48 hours of continuous cisatracurium infusion compared with the placebo group.
How do NMBAs work in ARDS?
NMBAs induce reversible muscle paralysis. By paralyzing the diaphragm, NMBAs associated with sedation could be responsible for the occurrence of lung atelectasis. This has especially been investigated in patients with healthy lungs in whom atelectasis rapidly occurs after anaesthesia with muscular paralysis (74). However, these findings were not observed in ARDS patients in whom NMBAs improve oxygenation and probably favour recruitment. One can suppose that the application of a sufficient PEEP level could counterbalance the effects of the loss of diaphragmatic tone in ARDS patients (75).
NMBAs improve the mechanical viscoelastic properties of the chest wall (59). An abolition in spontaneous ventilatory activity is accompanied by an increase in total thoraco-pulmonary compliance due to the better adaptation of the ventilator and a reduction in the expiratory muscular activity (7). The change in pulmonary ventilation/perfusion ratios induced by NMBAs could also be responsible for improving gas exchange, as much in terms of oxygenation (reduction of the zones with a low ventilation/perfusion ratio or West zone 3) as in terms of CO2 elimination (reduction of the physiologic dead space). Furthermore, an increase in the thoraco-pulmonary compliance in ARDS can increase the functional residual capacity (FRC) and decrease the intrapulmonary shunt (76). Finally, a modification in the ventilation/perfusion relationship could be related to a more homogenous redistribution of pulmonary perfusion enabled by the application of lower pulmonary pressures, thus favouring the perfusion of the ventilated zones. Muscle paralysis could also change the ventilatory distribution independent of the FRC and MV modifications by homogenizing the distribution of the delta-FRC linked to the PEEP and tidal volume.
One hypothesis to explain the beneficial effects of NMBAs during the early phase of ARDS is that by paralyzing the respiratory muscles, NMBAs minimize the manifestations of ventilator induced lung injury (VILI) with a reduction in the barotrauma, volutrauma, and atelectrauma and, subsequently, the biotrauma (77) (“respiratory muscles paralysis” hypothesis). The ventilatory strategy influences the pulmonary and systemic production of inflammatory mediators, and protective ventilation strategies are associated with a reduced release of pro-inflammatory agents (78,79). This hypothesis was explored in a controlled randomized study by Forel et al. (51) that analysed the effects of NMBAs on the systemic and pulmonary inflammatory responses in patients presenting with ARDS and ventilated with a protective ventilation strategy. A decrease in the interleukine (IL)-8 concentrations over time was observed in the pulmonary compartment of the NMBA group. Forty-eight hours after randomization, the pulmonary concentrations of IL-1β, IL-6 and IL-8 were lower in the NMBA group compared with the control group. A decrease over time in the IL-6 and IL-8 serum concentrations was also observed in the NMBA group. Forty-eight hours after randomization, the serum concentrations of IL-1β and IL-6 were lower in the NMBA group compared with the control group. Consequently, the early use of NMBAs decreases the pro-inflammatory response associated with ARDS and MV.
Nevertheless, cisatracurium could have a direct anti-inflammatory effect by inhibiting nicotinic acetylcholine receptor α1 (nAChRα1). In a recent animal study (80), Fanelli et al. compared, in an ARDS model, lung inflammation after the infusion of cisatracurium or pancuronium. In the cisatracurium and pancuronium groups, using the same lung protective ventilation, the authors observed a reduction in the pro-inflammatory cytokines with NMBAs compared with no NMBAs. An elegant experimental protocol shows that this reduction of inflammation is related to the inhibition of the nicotinic acetylcholine receptor-α1. Fanelli et al. conclude that the use of NMBA is lung protective through its anti-inflammatory properties by blocking the nAChRα1. Interestingly, a decrease in the biotrauma, namely, the release of pulmonary and systemic inflammatory mediators, could reduce the risk of multi organ dysfunction (77). In fact, it is impossible to differentiate between the beneficial effects of NMBAs associated with the reduction of biotrauma and a proper anti-inflammatory effect of cisatracurium.
The “respiratory muscles paralysis” hypothesis has been recently updated with new data. Recent experimental (81) and clinical (82) data using oesophageal pressure monitoring showed an advantage to continuous infusion of NMBAs in ARDS patients, which suppressed any spontaneous breathing. In an experimental ARDS rabbit model, Yoshida et al. (81) compared the effects of spontaneous breathing versus paralysis on the transpulmonary pressure and lung recruitment. They observed that spontaneous breathing in injured lungs increased the transpulmonary pressure during pressure control in volume-controlled ventilation, and spontaneous effort caused greater inflation and tidal recruitment of dorsal regions (>2-fold) versus during muscle paralysis, despite the same tidal volume and transpulmonary pressure. This phenomenon was caused by higher local lung stress; it should be noted that a plateau pressure inferior to 30 cmH2O does not mean that the local transpulmonary pressure was higher. Figure 1 summarizes the change in transpulmonary pressure during MV with or without spontaneous inspiratory efforts. Interestingly, Papazian et al. (2) reported an increased proportion of pneumothoraxes in the placebo patient group (11.7%, vs. 4.0% in the cisatracurium group; P=0.01). These pneumothoraxes appeared earlier in the placebo group. Recently, Mauri et al. (83) showed the possibility of high inspiratory transpulmonary pressure (due to remarkably negative swings in alveolar pressures) in a patient presenting with a severe ARDS under veno-venous ECMO and pressure support ventilation (PSV), while the tidal volume and plateau pressure were apparently safe (83). Recently, Guervilly et al. (82) randomized moderate ARDS patients in two groups, cisatracurium and placebo groups, and applied lung protective ventilation. The oesophageal pressure was monitored. The mean inspiratory and expiratory transpulmonary pressures were higher in the moderate ARDS group receiving NMBAs than in the control group. By contrast, there was no change in the driving pressure related to NMBA administration. In the NMBA group, the inspiratory transpulmonary pressure was lower than in the placebo group, suggesting tidal recruitment. The expiratory transpulmonary pressure was higher and positive in the NMBA group, which reflected a reduction of atelectrauma and expiratory derecruitment. By preventing active expiration, NMBA may allow the better control of PEEP (82). Atelectrauma could be reduced by NMBAs and paralysis minimizing the phenomena of repetitive opening and closing of the lung unit.
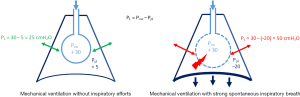
In a recent review, Brochard et al. (84) described the deleterious effects of spontaneous breathing (during MV) in a period of intense pulmonary inflammation similar to that in early ARDS. A non-application of protective ventilation can generate patient self-inflicted lung injury (P-SILI). These lung injuries are principally caused by a high level of transpulmonary pressure, resulting in increased transmural pulmonary vascular pressure (pulmonary oedema by increasing the alveolar-capillary permeability due to over-distention). These phenomena worsen the risk of pulmonary oedema through vascular leakage. The alteration of the haematosis caused by the aggravation of oedema will increase the respiratory drive and lead to self-aggravation.
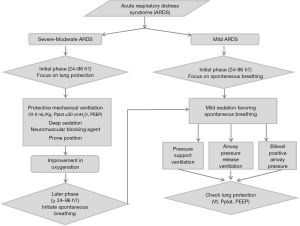
These data argue for limiting SV at the early phase of ARDS and support the use of deep sedation with NMBAs in severe to moderate ARDS. This early phase of ARDS is characterized by an intense lung pro-inflammatory state in which superimposed VILI is deleterious. To date, no routine clinical or paraclinical sign has indicated the duration of this “early inflammatory period”. The most relevant ARDS severity parameter is the PaO2/FiO2 ratio. We suggest the use of a 24- to 96-hour period of deep sedation and neuromuscular blockage in moderate to severe ARDS. In mild ARDS (PaO2/FiO2 ratio between 200 and 300 mmHg), we suggest that deep sedation and neuromuscular blockage should be avoided.
After the early inflammatory period of ARDS, sedation must be reduced to allow for spontaneous breathing. However, ICU physicians should ensure that the ventilation mode warrants a lung protective ventilation strategy. We emphasize that after the first 48 hours, NMBAs were stopped in the patients enrolled in the ACURASYS study (2). Moreover, sedation was titrated to allow SV (pressure support) as soon as the FiO2 reached less than 60%. To the best of our knowledge, the literature data are sparse and lack a RCT, and no definitive conclusions can be drawn. The BiRDS (NCT 01862016) study could bring interesting results. In the meantime, BIPAP™ or APRV, as suggested by Putensen et al. (40), could allow spontaneous breathing. Nevertheless, controlling the tidal volume and plateau pressure during the “spontaneous ventilation mode” is a key determinant of the outcome. The data reported by Yoshida (81) and Brochard (84) suggest that APRV will be more interesting than BIPAP™ or PSV. Indeed, the absence of synchronization of insufflation during APRV limits the risk of a high transpulmonary inspiratory pressure. As mentioned above, the modality and timing to switch from ACV to a MV mode, thus allowing spontaneous breathing, remains of interest for future investigations.
Even if their exact mechanism of action is not yet clearly identified, it is probable that several mechanisms contribute to the beneficial action of NMBAs in the early phase of ARDS.
Conclusions
Sedation and neuromuscular blockage are two contributors to the management of ARDS that are complementary instead of antagonistic. In the early management of ARDS, a protective ventilation strategy is essential and there may be a significant benefit to applying systematic muscular paralysis in the first 48 hours of ARDS. In this case, deep sedation must be applied, and the sedation score could be completed by BIS monitoring. At the acute phase of severe ARDS, one major goal is to limit VILI and favour lung recruitment by using a short course of NMBAs. Preserving spontaneous breathing during this time could generate P-SILI and aggravate lung injury. In a second phase of management, after this inflammatory period, the promotion of SV is a priority, and cooperative sedation could be useful for weaning MV and reducing the length of MV. However, the ideal duration of deep sedation and pharmacological paralysis remains unknown. The principles of protective ventilation must be maintained when initiating SV with a reduction in the tidal volume and plateau pressure. Intense inspiratory efforts can generate high levels of inspiratory transpulmonary pressure observed on oesophageal pressure monitoring. The incidence of a high level of inspiratory transpulmonary pressure is correlated with the severity of P-SILI. To address these issues, we propose an algorithm to help clinicians manage sedation and paralysis during ARDS (Figure 2).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Desbiens NA, Wu AW, Broste SK, et al. Pain and satisfaction with pain control in seriously ill hospitalized adults: findings from the SUPPORT research investigations. For the SUPPORT investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatmentm. Crit Care Med 1996;24:1953-61. [Crossref] [PubMed]
- Puntillo KA, Arai S, Cohen NH, et al. Symptoms experienced by intensive care unit patients at high risk of dying. Crit Care Med 2010;38:2155-60. [Crossref] [PubMed]
- Turner JS. On the thermal capacity of a bird's egg warmed by a brood patch. Physiol Zool 1997;70:470-80. [Crossref] [PubMed]
- Chanques G, Kress JP, Pohlman A, et al. Impact of ventilator adjustment and sedation-analgesia practices on severe asynchrony in patients ventilated in assist-control mode. Crit Care Med 2013;41:2177-87. [Crossref] [PubMed]
- Coggeshall JW, Marini JJ, Newman JH. Improved oxygenation after muscle relaxation in adult respiratory distress syndrome. Arch Intern Med 1985;145:1718-20. [Crossref] [PubMed]
- Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471-7. [Crossref] [PubMed]
- Schweickert WD, Gehlbach BK, Pohlman AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med 2004;32:1272-6. [Crossref] [PubMed]
- Davidson TA, Caldwell ES, Curtis JR, et al. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA 1999;281:354-60. [Crossref] [PubMed]
- Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013;39:1704-13. [Crossref] [PubMed]
- Davydow DS, Gifford JM, Desai SV, et al. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med 2009;35:796-809. [Crossref] [PubMed]
- Schelling G, Stoll C, Haller M, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med 1998;26:651-9. [Crossref] [PubMed]
- Wade D, Hardy R, Howell D, et al. Identifying clinical and acute psychological risk factors for PTSD after critical care: a systematic review. Minerva Anestesiol 2013;79:944-63. [PubMed]
- Davydow DS, Desai SV, Needham DM, et al. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med 2008;70:512-9. [Crossref] [PubMed]
- Samuelson KA, Lundberg D, Fridlund B. Stressful memories and psychological distress in adult mechanically ventilated intensive care patients—a 2-month follow-up study. Acta Anaesthesiol Scand 2007;51:671-8. [Crossref] [PubMed]
- Jones C, Backman C, Capuzzo M, et al. Precipitants of post-traumatic stress disorder following intensive care: a hypothesis generating study of diversity in care. Intensive Care Med 2007;33:978-85. [Crossref] [PubMed]
- Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med 2015;41:2130-7. [Crossref] [PubMed]
- Sackey PV, Martling CR, Carlsward C, et al. Short- and long-term follow-up of intensive care unit patients after sedation with isoflurane and midazolam--a pilot study. Crit Care Med 2008;36:801-6. [Crossref] [PubMed]
- Wade DM, Howell DC, Weinman JA, et al. Investigating risk factors for psychological morbidity three months after intensive care: a prospective cohort study. Crit Care 2012;16:R192. [Crossref] [PubMed]
- Bienvenu OJ, Gellar J, Althouse BM, et al. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med 2013;43:2657-71. [Crossref] [PubMed]
- Treggiari MM, Romand JA, Yanez ND, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med 2009;37:2527-34. [Crossref] [PubMed]
- Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med 2010;182:183-91. [Crossref] [PubMed]
- Bugedo G, Tobar E, Aguirre M, et al. The implementation of an analgesia-based sedation protocol reduced deep sedation and proved to be safe and feasible in patients on mechanical ventilation. Rev Bras Ter Intensiva 2013;25:188-96. [Crossref] [PubMed]
- Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263-306. [Crossref] [PubMed]
- Simmons LE, Riker RR, Prato BS, et al. Assessing sedation during intensive care unit mechanical ventilation with the Bispectral Index and the Sedation-Agitation Scale. Crit Care Med 1999;27:1499-504. [Crossref] [PubMed]
- Roizen MF, Saidman LJ. Redefining anesthetic management. Goals for the anesthesiologist. Anesthesiology 1994;80:251-2. [Crossref] [PubMed]
- Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003;289:2983-91. [Crossref] [PubMed]
- Hernández-Gancedo C, Pestaña D, Peña N, et al. Monitoring sedation in critically ill patients: bispectral index, Ramsay and observer scales. Eur J Anaesthesiol 2006;23:649-53. [Crossref]
- Trouiller P, Fangio P, Paugam-Burtz C, et al. Frequency and clinical impact of preserved bispectral index activity during deep sedation in mechanically ventilated ICU patients. Intensive Care Med 2009;35:2096-104. [Crossref] [PubMed]
- Bilgili B, Montoya JC, Layon AJ, et al. Utilizing bi-spectral index (BIS) for the monitoring of sedated adult ICU patients: a systematic review. Minerva Anestesiol 2017;83:288-301. [PubMed]
- Torbic H, Bauer SR, Personett HA, et al. Perceived safety and efficacy of neuromuscular blockers for acute respiratory distress syndrome among medical intensive care unit practitioners: A multicenter survey. J Crit Care 2017;38:278-83. [Crossref] [PubMed]
- Errando CL, Sigl JC, Robles M, et al. Awareness with recall during general anaesthesia: a prospective observational evaluation of 4001 patients. Br J Anaesth 2008;101:178-85. [Crossref] [PubMed]
- Domino KB, Posner KL, Caplan RA, et al. Awareness during anesthesia: a closed claims analysis. Anesthesiology 1999;90:1053-61. [Crossref] [PubMed]
- Sedation in French intensive care unit. Ann Intensive Care 2013;3:24. [Crossref] [PubMed]
- Payen JF, Chanques G, Mantz J, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology 2007;106:687-95. [Crossref] [PubMed]
- Jabaudon M, Boucher P, Imhoff E, et al. Sevoflurane for Sedation in Acute Respiratory Distress Syndrome. A Randomized Controlled Pilot Study. Am J Respir Crit Care Med 2017;195:792-800. [Crossref] [PubMed]
- Conti G, Ranieri VM, Costa R, et al. Effects of dexmedetomidine and propofol on patient-ventilator interaction in difficult-to-wean, mechanically ventilated patients: a prospective, open-label, randomised, multicentre study. Crit Care 2016;20:206. [Crossref] [PubMed]
- Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 2012;307:1151-60. [Crossref] [PubMed]
- Putensen C, Mutz NJ, Putensen-Himmer G, et al. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1999;159:1241-8. [Crossref] [PubMed]
- Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care 2001;5:221-6. [Crossref] [PubMed]
- Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327-35. [Crossref] [PubMed]
- Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011;183:364-71. [Crossref] [PubMed]
- Wrigge H, Zinserling J, Neumann P, et al. Spontaneous breathing with airway pressure release ventilation favors ventilation in dependent lung regions and counters cyclic alveolar collapse in oleic-acid-induced lung injury: a randomized controlled computed tomography trial. Crit Care 2005;9:R780-9. [Crossref] [PubMed]
- Yoshida T, Rinka H, Kaji A, et al. The impact of spontaneous ventilation on distribution of lung aeration in patients with acute respiratory distress syndrome: airway pressure release ventilation versus pressure support ventilation. Anesth Analg 2009;109:1892-900. [Crossref] [PubMed]
- Henzler D, Hochhausen N, Bensberg R, et al. Effects of preserved spontaneous breathing activity during mechanical ventilation in experimental intra-abdominal hypertension. Intensive Care Med 2010;36:1427-35. [Crossref] [PubMed]
- Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646-55. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004;32:113-9. [Crossref] [PubMed]
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006;34:2749-57. [Crossref] [PubMed]
- Rhoney DH, Murry KR. National survey of the use of sedating drugs, neuromuscular blocking agents, and reversal agents in the intensive care unit. J Intensive Care Med 2003;18:139-45. [Crossref] [PubMed]
- Christensen BV, Thunedborg LP. Use of sedatives, analgesics and neuromuscular blocking agents in Danish ICUs 1996/97. A national survey. Intensive Care Med 1999;25:186-91. [Crossref] [PubMed]
- Arroliga A, Frutos-Vivar F, Hall J, et al. Use of sedatives and neuromuscular blockers in a cohort of patients receiving mechanical ventilation. Chest 2005;128:496-506. [Crossref] [PubMed]
- Arroliga AC, Thompson BT, Ancukiewicz M, et al. Use of sedatives, opioids, and neuromuscular blocking agents in patients with acute lung injury and acute respiratory distress syndrome. Crit Care Med 2008;36:1083-8. [Crossref] [PubMed]
- Murray MJ, DeBlock H, Erstad B, et al. Clinical Practice Guidelines for Sustained Neuromuscular Blockade in the Adult Critically Ill Patient. Crit Care Med 2016;44:2079-103. [Crossref] [PubMed]
- Samuelson KA, Larsson S, Lundberg D, et al. Intensive care sedation of mechanically ventilated patients: a national Swedish survey. Intensive Crit Care Nurs 2003;19:350-62. [Crossref] [PubMed]
- Vender JS, Szokol JW, Murphy GS, et al. Sedation, analgesia, and neuromuscular blockade in sepsis: an evidence-based review. Crit Care Med 2004;32:S554-61. [Crossref] [PubMed]
- Hunter JM. New neuromuscular blocking drugs. N Engl J Med 1995;332:1691-9. [Crossref] [PubMed]
- Murray MJ, Cowen J, DeBlock H, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med 2002;30:142-56. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004;126:518-27. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017;45:486-552. [Crossref] [PubMed]
- Conti G, Vilardi V, Rocco M, et al. Paralysis has no effect on chest wall and respiratory system mechanics of mechanically ventilated, sedated patients. Intensive Care Med 1995;21:808-12. [Crossref] [PubMed]
- Lagneau F, D'Honneur G, Plaud B, et al. A comparison of two depths of prolonged neuromuscular blockade induced by cisatracurium in mechanically ventilated critically ill patients. Intensive Care Med 2002;28:1735-41. [Crossref] [PubMed]
- Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011;10:931-41. [Crossref] [PubMed]
- De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002;288:2859-67. [Crossref] [PubMed]
- Witt NJ, Zochodne DW, Bolton CF, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest 1991;99:176-84. [Crossref] [PubMed]
- Sharshar T, Bastuji-Garin S, Stevens RD, et al. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med 2009;37:3047-53. [Crossref] [PubMed]
- Weber-Carstens S, Deja M, Koch S, et al. Risk factors in critical illness myopathy during the early course of critical illness: a prospective observational study. Crit Care 2010;14:R119. [Crossref] [PubMed]
- Griffiths RD, Hall JB. Intensive care unit-acquired weakness. Crit Care Med 2010;38:779-87. [Crossref] [PubMed]
- Hansen-Flaschen J, Cowen J, Raps EC. Neuromuscular blockade in the intensive care unit. More than we bargained for. Am Rev Respir Dis 1993;147:234-6. [Crossref] [PubMed]
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013;17:R43. [Crossref] [PubMed]
- Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 2006;32:24-33. [Crossref] [PubMed]
- Hedenstierna G, Strandberg A, Brismar B, et al. Functional residual capacity, thoracoabdominal dimensions, and central blood volume during general anesthesia with muscle paralysis and mechanical ventilation. Anesthesiology 1985;62:247-54. [Crossref] [PubMed]
- Tokics L, Hedenstierna G, Svensson L, et al. V/Q distribution and correlation to atelectasis in anesthetized paralyzed humans. J Appl Physiol (1985) 1996;81:1822-33. [PubMed]
- Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 1998;157:1721-5. [Crossref] [PubMed]
- Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med 1999;160:109-16. [Crossref] [PubMed]
- Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005;33:1-6; discussion 230-2. [Crossref] [PubMed]
- Fanelli V, Morita Y, Cappello P, et al. Neuromuscular Blocking Agent Cisatracurium Attenuates Lung Injury by Inhibition of Nicotinic Acetylcholine Receptor-alpha1. Anesthesiology 2016;124:132-40. [Crossref] [PubMed]
- Yoshida T, Nakahashi S, Nakamura MA, et al. Volume Controlled Ventilation Does Not Prevent Injurious Inflation During Spontaneous Effort. Am J Respir Crit Care Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Guervilly C, Bisbal M, Forel JM, et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med 2017;43:408-18. [Crossref] [PubMed]
- Mauri T, Langer T, Zanella A, et al. Extremely high transpulmonary pressure in a spontaneously breathing patient with early severe ARDS on ECMO. Intensive Care Med 2016;42:2101-3. [Crossref] [PubMed]
- Brochard L. Ventilation-induced lung injury exists in spontaneously breathing patients with acute respiratory failure Yes. Intensive Care Med 2017;43:250-2. [Crossref] [PubMed]


