Definition and epidemiology of acute respiratory distress syndrome
Introduction
Acute respiratory distress syndrome (ARDS) is an acute inflammatory lung process, which leads to protein-rich non-hydrostatic pulmonary edema, causes refractory hypoxemia, increases lung “stiffness” and impairs the ability of the lung to eliminate carbon dioxide.
At a macroscopical level, computed-tomography (CT) studies gave birth to the fascinating concept of “baby lung”, suggesting a change of perspective from a “stiff” to a “small” lung (1). Furthermore, the CT study of the gravity effect on the lungs, using the prone position, helped to better understand how the “baby lung” was not an anatomical and static concept, but a functional one, visualized in a “sponge” model (2). High dead space fraction was discovered to be correlated to increased mortality in ARDS patients (3). In other words, the higher was the amount of lungs that did not participate to gas exchanges, the higher was the proportion of ARDS mortality. The continuous efforts aimed to improve the ARDS definition, together with the advanced diagnostic tools and therapeutic strategies, had an impact on the ability to recognize the onset of ARDS and to change the clinical history of ARDS. In this review, we will provide the readers the essential information to understand the process leading to the new Berlin definition and the change of ARDS epidemiology over 50 years since original Ashbaugh’s definition of ARDS.
History of ARDS: the long path to define a syndrome—new acquisitions and limitations
The first description of ARDS probably belongs to Laennec, who defined it as “idiopathic pulmonary edema” in 1821 (4). The following century saw a number of traumatic injuries during the “big wars” period that eventually earned the definition of “wet lung” or “shock lung” to the unexplained lung edema (5,6). However, it was only in 1967 that Ashbaugh and colleagues termed, for the first time, “ARDS” a syndrome characterized by “acute onset of tachypnoea, hypoxaemia, and loss of compliance after a variety of stimuli” (7).
Ashbaugh et al. reported the presence of a specific clinical presentation seen in 12 adult patients and characterized by severe dyspnea and tachypnea, cyanosis not responsive to oxygen therapy, loss of lung compliance and presence of diffuse alveolar infiltration at chest X-ray evaluation, with a high mortality rate.
Since then, ARDS paradigm included the presence of a known risk factor for ARDS, severe hypoxemia despite high FiO2 delivery, bilateral pulmonary infiltrates and the exclusion of cardiogenic edema as a cause.
From the first ARDS definition, 50 years ago, different efforts have been dedicated to clarify the pathophysiology and the severity of this multifaceted syndrome.
In 1988, Murray and colleagues proposed a definition known as “expanded definition of ARDS”. The authors took into account four different variables to which they assigned a score [0–4]: (I) the chest roentgenogram score, that describes the amount of pulmonary consolidation of the four quadrants; (II) the hypoxaemia stratified according to PaO2/FiO2 classes; (III) the PEEP level; and (IV) the compliance of the respiratory system. The final score, called “the Murray Lung Injury Score”, is calculated as the sum of the single components score divided by the number of the accounted variables, and defines three categories: absence of lung injury (0), mild to moderate lung injury (1–2.5), and severe lung injury (>2.5) (8).
In 1994, the American-European Consensus Conference (AECC) defined acute lung injury (ALI) and ARDS as respiratory failure with: (I) acute onset; (II) presence of bilateral infiltrates at the chest X-ray; (III) pulmonary wedge pressure ≤18 mmHg or no clinical evidence of high left atrial pressure (to rule out a cardiogenic cause of lung edema); and (IV) hypoxemia, independently of the PEEP level. The severity of hypoxia, defined the class of lung injury as ALI (PaO2/FiO2 ≤300) or ARDS (PaO2/FiO2 ≤200) (9).
Compared to the Murray Score, AECC definition of ARDS was characterized by the exclusion of a cardiogenic cause of edema, but it did not include the respiratory system compliance calculation at the diagnosis, and did not mention the PEEP level set.
Since respiratory failure due to ARDS is not just typical of the adult population but it is represented also in infants, AECC definition was clinically used either in adult or in pediatric critical care (10-12) to contextualize the grade of lung injury. However, oxygenation index (OI), proposed for the first time by Dr. Bartlett studying indications for ECMO in neonates with respiratory failure (13,14), is a commonly accepted indicator to describe lung injury in the pediatric population. OI is calculated as the product of mean airway pressure (in mmHg) × FiO2 (in percent)/arterial partial pressure of oxygen (in mmHg).
In 2005, a group of experts from the University of Toronto proposed a formal consensus method to improve the AECC ARDS definition, using the Delphi technique (15). The Delphi technique consists in an individual survey of the participating panelists who anonymously receive group comments between iterations. The novelty of this approach included a clear definition of the acuity of the respiratory failure (<72 hours), the standardization of hypoxemia (PaO2/FiO2 ≤200 setting a threshold of PEEP level ≥10 cmH2O), the inclusion of the static respiratory system compliance (calculated with a tidal volume of 8 mL/kg of ideal body weight in a sedated patient with a PEEP level ≥10 cmH2O), and the presence of an ARDS predisposing factor (pulmonary versus extrapulmonary ARDS). Radiographic abnormalities introduced the concept of airspace disease involving ≥2 quadrants on frontal chest X-ray, and the role of echocardiography was mentioned to exclude a possible cardiogenic origin of the lung edema, under clinical indication.
In 2013, Villar and colleagues, proposed a refinement of the classification of severity of ARDS, aimed at assessing the ICU mortality risk, according to the PaO2/FiO2 ratio. The authors measured PaO2/FiO2 ratio at ARDS onset and 24 hours later, testing two different combinations of PEEP (≥5 and ≥10 cmH2O) and FiO2 (≥0.5 and 1.0). The better ARDS risk stratification was obtained setting PEEP ≥10 cmH2O and FiO2 ≥0.5 at 24 hours after ARDS diagnosis, with mortality rates increasing from 17%, to 40.9%, to 58.1%, in mild, moderate and severe ARDS, respectively (16).
After 18 years of AECC definition, the need of a new definition of ARDS with more specific and generalizable criteria emerged.
Hence, the most recent revisited definition of ARDS was proposed by a “task force” endorsed by the European Society of intensive Care Medicine, and it is now known as the “Berlin definition” of ARDS (17).
Compared to the AECC definition, the ARDS Berlin definition clarified: (I) the acute onset, established within one week; (II) the characteristics of the bilateral lung infiltrates, on chest X-ray or CT scan; (III) the source of lung edema, without including a pulmonary capillary wedge pressure cut-off; (IV) the standardization of the hypoxemia, calculated with a PEEP level ≥5 cmH2O, and the categorization of the lung injury into three grades of severity according to the PaO2/FiO2 ratio; furthermore, (V) if no predisposing condition was identified, as specified also in the Delphi consensus, an “objective” evaluation is mandated to rule out the cardiogenic origin of the lung edema. Berlin definition does not separate anymore, as the AECC definition of ARDS did, ALI and ARDS, unifying ARDS definition in a single entity graded into three classes of severity, according to the PaO2/FiO2 ratio and with a necessary minimum amount of PEEP (5 cmH2O). This important step led not to overestimate patient hypoxemia just caused by lung atelectasis and then easily reversible with minimal PEEP (18). Consequently, ARDS definition could be applied to a less heterogeneous population, excluding patients with compromised oxygenation only in the absence of PEEP. The classification of ARDS into three increasing stages of severity—mild, moderate, and severe—according to the level of hypoxemia, significantly reflected an increased mortality rate (respectively: 27%, 95% CI, 24–30%; 32%, 95% CI, 29–34%; and 45%, 95% CI, 42–48%). Compared to AECC definition, predictive validity for mortality was significantly improved in the Berlin definition of ARDS (area under the ROC, 0.577, 95% CI, 0.561–0.593 vs. 0.536, 95% CI, 0.520–0.553).
Berlin definition was proved to be adaptable also for pediatrics (19). De Luca et al., on behalf of the Respiratory Section of the European Society for Pediatric and Neonatal Intensive Care, in a retrospective, international, multicenter study of infants and early children with ALI or ARDS—according to the AECC definition—reported that the predictive validity for ARDS mortality using the Berlin definition was confirmed and comparable to the results showed in adult population (17). This finding was mainly correlated to the introduction of the new category of “severe ARDS”, which demonstrated a higher mortality among the Berlin definition classes of ARDS. These results have been further recently confirmed by Barreira et al. (20).
Still, despite the contributions of several scientists, an entirely satisfactory definition of ARDS proofed to be an elusive goal.
The principal hindrance is due to the intrinsic nature of ARDS which is not a disease, with a univocal and straightforward trajectory of treatment and recovery, but a syndrome, composed of a multifaceted means of diagnosis and determined by different causes, with as many different clinical histories. Moreover, the pathological hallmark of ARDS (i.e., noncardiogenic pulmonary edema) cannot be easily identified by current clinical tool, and any clinical definition must rely on accessible proxies (e.g., hypoxaemia, chest X-ray).
The reliability of hypoxia definition is still controversial. Measurements of PaO2 varies on time, PEEP level, and FiO2 (16,21-24).
The chest X-ray, used to classify ARDS based on the presence of bilateral infiltrates, is not completely accurate, either for a relevant interobserver variability (25,26) or if compared to other diagnostic imaging tests, such as ultrasonography (27) and computed tomography (28). Promising diagnostic advances have been recently proposed, using ultrasound to rule out a cardiogenic source of lung edema and to predict ARDS in blunt trauma patients (29-31), and using low-dose chest CT to monitor and redirect the treatment strategy of the ventilator setting (32,33). Along with measurements of SpO2/FiO2 by pulse-oximetry, in lack of PaO2/FiO2 measurements (34), lung ultrasound (35) can be extremely appealing in settings with limited ICU resources, as lately proposed by the Kigali modification of the Berlin definition (36).
Etiology of ARDS and ARDS phenotypes
ARDS is the result of a wide spectrum of different risk factors, which can be either local or systemic (37).
ARDS can be classified according to the origin of the inflammatory insult as direct lung insult or indirect lung injury.
The first one leads to the commonly known “pulmonary ARDS” (ARDSp), the second one to the “extrapulmonary ARDS” (ARDSexp) (38,39) (Table 1).

Full table
While pneumonia, extrapulmonary sepsis, and aspiration are the most frequent clinical risk factors for ARDS (40,41), chronic diseases such as obesity (42,43) and diabetes (44,45) have been associated to a lower occurrence of ARDS. The “obesity paradox”, as called in a recent meta-analysis of the literature (42), is still hard to understand, due to the lack of a clear pathophysiologic mechanism behind these findings (46), and with some conflicting preclinical data (47). On the other side, instead, diabetes might be protective by means of a depressed immune response of the organism against an inflammatory insult (44). Among the modifiable risk factors for ARDS, alcohol abuse emerges (48,49) and impaired immune response involving alveolar macrophages is reported (50,51). Since the observation that positive cumulative fluid balance is independently associated to higher mortality rate in patients with lung injury (52), a number of study raised in order to assess the impact of conservative fluid management or active fluid removal on mortality in ARDS patients, but results are yet not conclusive (53,54).
High occurrence of ARDS has been linked also to demographic and environmental risk factors. These include older age (55), non-Caucasian race (56), defined genetic variants (57) and ozone exposure (58).
Some authors, studying patients with a number of predisposing conditions of ARDS and with associated risk factors for the development of ARDS, conceived and validated the acute lung injury prediction score (LIPS). However, the best LIPS score cut-off was able to predict ARDS with sub-optimal sensitivity (69%) and specificity (78%) (59).
Recently, thanks to an analysis of clinical and laboratory data from two large randomized clinical trials (RCTs)—the ARMA (60-62) and the ALVEOLI trials (63)—the ARDS Network identified two different phenotypes of ARDS. Phenotype 2—named hyperinflammatory subphenotype—is characterized by higher prevalence of inflammation, shock, sepsis and metabolic acidosis than phenotype 1.
The relevance of the ARDS subphenotypes classification showed not just a difference about clinical and laboratory data between the two clinical conditions, but more important, that subphenotype 2 recognizes a cohort of patients with worse clinical outcome, and with higher mortality, than phenotype 1 (64). A further analysis by the same group of the Fluid and Catheter Treatment Trial (FACTT) simplified the 2 phenotypes using a three-variable model (IL-8, bicarbonate and tumor necrosis factor receptor-1) that had a different answer in terms of fluid management strategy. In other words, ARDS subphenotypes can predict the severity of the disease and can direct the treatment choice (65).
Pathology of ARDS
As stated, diffuse alveolar damage (DAD) leading to high permeability pulmonary edema is considered the histopathologic hallmark of ARDS (66-69). For this reason, while a “prefect” bedside definition of ARDS should capture all patients with DAD, without false positives, this is not always the case. This was evident since the first description by Ashbaugh and colleagues, later proved by the group of Vincent JL (70). More recently, these findings were further confirmed by Guerin and colleagues, studying patients with non-resolving ARDS (71).
The authors reported that DAD was markedly represented in non-resolving ARDS with no difference among the increasing level of ARDS severity, according to the Berlin definition. The presence of DAD, at the pathology examination, has also a relevant role to identify the clinical history of the disease.
In a recent meta-analysis of lung biopsy series for patients with ARDS, the presence of a pathologic pattern of DAD was associated with higher mortality compared to ARDS with no DAD (72).
ARDS is pathologically categorized into acute, subacute and chronic phase (73,74). The acute phase—exudative—(within 6 days) see the presence of either interstitial or alveolar edema, with acute inflammatory cells and red blood cells into the alveoli. Both endothelial and epithelial layers are damaged, and hyaline membranes develop in the alveoli. The subacute phase—proliferative—(between 7–14 days) shows the reabsorption of the edema, the proliferation of the alveolar epithelial type II cells and the fibroblastic infiltration with deposition of collagen fibers. The chronic stage—fibrotic—(following 14 days) presents the clearance of the neutrophils, the abundance of alveolar mononuclear cells and macrophages into the alveoli and marked fibrosis, with a repairing process involving the alveolar epithelium.
ARDSp and ARDSexp are two different features of ARDS, not just in terms of etiology, but also regarding the characteristics of the lung lesions. Of note, ARDSp is characterized by the more pronounced alveolar collapse, fibrinous exudative material and edema of the alveolar walls, compared to ARDSexp (75). Furthermore, ARDSp has an increased collagen content, with a prevalence of the extracellular matrix remodeling (76).
Epidemiology
ARDS incidence—an underestimated syndrome
Since the beginning of the recognition of ARDS as defined entity, several studies tried to provide essential information about ARDS epidemiology. Most of them were constructed following the AECC definitions.
It is surprising the huge variability of ARDS incidence, including all ARDS categories, in various population-based studies (77) between different continents such as South America (10.1 per 100,000 person-years) (78), Europe, (17.9 per 100,000 person-years) (79), Australia (34 per 100,000 person-years) (80) and USA (78.9 per 100,000 person-years) (81) with a relevant geographic diversity. Furthermore, in countries of the same continent such as Europe, ARDS occurrence varies consistently, ranging from 10.6 per 100,000 person-years in Finland (82), to 17.9 per 100,000 person-years in Scandinavia (79), to 25.5 per 100,000 person-years in Spain (55). This is the case also for hospitalization based studies (77), which showed a ARDS proportion ranging from 7.1% (83) to 12.5% (84) of incidence proportion of all ICU admissions in Europe to 19% in 14 ICUs of Ireland (85), among the admitted patients (Table 2).

Full table
Data incidence of ARDS in pediatric population shows less variability between different continents such as Europe [2.2 per 100,000 person-years in the Netherlands (90) and 3.9 per 100,000 person-years in Spain (91)] and Australia [2.6 per 100,000 person-years (92)], with the highest ARDS incidence in USA with 9.5 ARDS cases per 100,000 person-years (93). However, the less relevant geographic difference of ARDS incidence in children has to be interpreted considering the lower ARDS incidence in pediatrics compared to adult population.
Differences in availability of diagnostic methodologies, health resources, hospital admission practices and emergency medicine networks clearly impact on the recognition of critical diseases. In a careful overview on the global burden of critical illness in adults, Rubenfeld and coworkers observe how the availability of ICU beds differs between high-income countries and less advantaged countries. This disproportion might lead, therefore, to different filtering of acute critical diseases—such as ALI—due to different levels of care, and to a wide discrepancy in incidence estimation (94).
Recent insights about the epidemiology of ARDS, according to the current Berlin definition, came from the LUNG SAFE study, an International, multicenter, prospective cohort study conducted in Intensive Care Units in 50 countries (41). Due to its design, the LUNG SAFE study cannot provide “population-based” estimates of ARDS incidence, or prevalence, but only ICU. ARDS occurrence was estimated to be 10.4% in all ICU admissions and in more than double (23.4%) among the mechanically ventilated patients. In a further analysis by country, the incidence of ARDS was the highest in Oceania, with 0.57 cases/ICU bed/year, followed by Europe, North America, Africa, South America and Asia, with the lowest ARDS occurrence of 0.27 cases/ICU bed/year. These findings are in line with previous epidemiologic studies (80,81,88,89,95,96) and might be interpreted in the light of the different distribution of ICU resources.
One of the most striking finding of the LUNG SAFE study was that of all ARDS patients, clinicians missed almost 40% of ARDS diagnosis, despite a specific online training on ARDS diagnosis, which was offered to all investigators. Even among severe ARDS, diagnosis was missed in at least one patient out of 5. These results are in line with another study that estimated ARDS under-recognition by clinicians in up to 50% of cases, despite the accepted use of AECC definition and staff training (55). In LUNG SAFE, organizational and patient factors were reported to be associated with higher clinician recognition of ARDS in invasively ventilated patients. Among the first ones, the authors observed higher nurse-to-patient and physician-to-patient ratios. Patient variables associated with a lower ARDS under-diagnosis were younger patient age, lower predicted body weight, higher non-pulmonary SOFA score, a lower PaO2/FIO2 ratio, and the presence of pneumonia, pancreatitis, neoplastic or immune or hematological disease, trauma at admission, absence of ARDS risk factors and concomitant presence heart failure.
ARDS clinical management
A second relevant finding of LUNG safe regards the therapeutic management of ARDS. Mechanical ventilation and therapeutic adjunctive measures to target ARDS are still not yet optimized, with significant potential future improvement. About 4 out of 5 patients were treated with a PEEP level below 12 cmH2O. Plateau pressure, a well-known parameter of respiratory mechanics associated with mortality in ARDS patients (60), was measured in only 40.1% of the ARDS population. Among patients with ARDS, about 1 out of 3 patients with ARDS did not receive protective mechanical ventilation, with either a plateau pressure above 30 cmH2O or a tidal volume above 8 mL/kg of predicted body weight. Large tidal volumes are far from a diffuse and accepted clinical practice, but they mirror the results showed in two recent RCTs (97,98). Furthermore, data suggest that clinicians seem more incline to adjust FiO2 than to increase PEEP to treat hypoxemia. Finally, adjunctive measures such as recruitment maneuvers and prone positioning were used in a minority of ARDS patients (20.9% and 7.9%, respectively) (41).
ARDS mortality—still a critical challenge
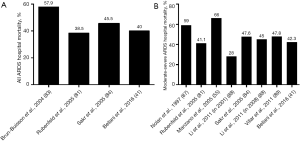
Mortality in ARDS patients is still high. The LUNG SAFE study reports a hospital mortality of 40%, with a significant increase across the ARDS severity categories, in line with Berlin definition (34.9%, in mild ARDS; 40.3% in moderate ARDS; 46.1% in severe ARDS). These results proof that the Berlin definition of ARDS is an excellent predictor of outcomes in the studied population. In this context, Laffey and colleagues examined the predictors associated with outcome in a secondary analysis of the LUNG SAFE study. In 2,377 ARDS patients, who received mechanical ventilation, the authors observed that lower ventilation pressure (peak, plateau and driving), higher PEEP level, and lower respiratory rate confirm their validity as predictors of improved survival from ARDS (99). These results are in line with data from previously observed clinical trials (60,63,100,101). ARDS mortality decreased over the years probably also thanks to the improved therapeutic management of the ventilatory settings (60,63,100-102). In the early 90s, Nolan and colleagues reported a hospital mortality of 59% in moderate and severe ARDS in a population based study conducted in Australia (87). Brun-Buisson et al. described similar findings in the late 90s, in a hospitalization based study conducted in 78 ICUs in Europe, with a proportion of 57.9% even including mild ARDS (83). Since then, ARDS hospital mortality decreased to a stable level of about 40%, including all ARDS categories (25,84), and of about 45% considering moderate-severe ARDS (84,88,89). These findings are corroborated by mortality data of the recent LUNG SAFE study (41) (Figure 1A,B). Moreover, data from two large population based study in northern European countries, analyzing patients with higher severity of ARDS (moderate and severe ARDS), reports a 90-day long term mortality among 38–41.2% (79,86), and when overall ARDS is analyzed, Linko et al. described a 90-day mortality of 47% (82). While in adult population ARDS has shown a clear trend of decrease in mortality over the last decades, these results are not univocally confirmed in pediatric population, as reported with conflicting results in two recent meta-analysis of the literature (103,104). Over the last few years, different RCTs have proposed promising results in terms of ARDS mortality improvement (53,105,106). Unfortunately, this enthusiasm has to be well weighted in sight of the study design (107,108). Enrolled subjects of RCTs are rigorously selected, and the generalization of the results from a RCT might be deceptive if applied to the entire population.
Conclusions
After 50 years of study, ARDS still looks nowadays a threatening enemy to defeat. Definition of ARDS has been improved over the years. Even so, the definition of hypoxemia in different settings and timing is debatable and the agreement about the optimal diagnostic imaging is yet to be reached. Studies on ARDS incidence consistently show that the disease is not rare, albeit often not recognized by clinicians. ARDS is undertreated and basic ventilator strategies are not yet standardly optimized, despite major advances in the management of mechanical ventilation and non-ventilatory strategies, aimed at preventing the VILI. Finally, ARDS mortality remains high. This suggests the need of a commonly accepted therapeutic strategy for ARDS, which should be prerogative of all the countries including the less developed ones.
Acknowledgements
This work was supported by institutional funds.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gattinoni L, Pesenti A. The concept of "baby lung". Intensive Care Med 2005;31:776-84. [Crossref] [PubMed]
- Gattinoni L, Pesenti A, Carlesso E. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure: impact and clinical fallout through the following 20 years. Intensive Care Med 2013;39:1909-15. [Crossref] [PubMed]
- Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002;346:1281-6. [Crossref] [PubMed]
- Laennec RT; Classics of Medicine Library. A treatise on the diseases of the chest, in which they are described according to their anatomical characters, and their diagnosis established on a new principle by means of acoustick instruments. Birmingham, AL: Classics of Medicine Library, 1979.
- Montgomery AB. Early description of ARDS. Chest 1991;99:261-2. [Crossref] [PubMed]
- Morris MJ. Acute respiratory distress syndrome in combat casualties: military medicine and advances in mechanical ventilation. Mil Med 2006;171:1039-44. [Crossref] [PubMed]
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2:319-23. [Crossref] [PubMed]
- Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988;138:720-3. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- Yu WL, Lu ZJ, Wang Y, et al. The epidemiology of acute respiratory distress syndrome in pediatric intensive care units in China. Intensive Care Med 2009;35:136-43. [Crossref] [PubMed]
- Randolph AG, Vaughn F, Sullivan R, et al. Critically ill children during the 2009-2010 influenza pandemic in the United States. Pediatrics 2011;128:e1450-8. [Crossref] [PubMed]
- Bindl L, Dresbach K, Lentze MJ. Incidence of acute respiratory distress syndrome in German children and adolescents: a population-based study. Crit Care Med 2005;33:209-312. [Crossref] [PubMed]
- Trachsel D, McCrindle BW, Nakagawa S, et al. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2005;172:206-11. [Crossref] [PubMed]
- Ortiz RM, Cilley RE, Bartlett RH. Extracorporeal membrane oxygenation in pediatric respiratory failure. Pediatr Clin North Am 1987;34:39-46. [Crossref] [PubMed]
- Ferguson ND, Davis AM, Slutsky AS, et al. Development of a clinical definition for acute respiratory distress syndrome using the Delphi technique. J Crit Care 2005;20:147-54. [Crossref] [PubMed]
- Villar J, Perez-Mendez L, Blanco J, et al. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting--a prospective, multicenter validation study. Intensive Care Med 2013;39:583-92. [Crossref] [PubMed]
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Caironi P, Carlesso E, Cressoni M, et al. Lung recruitability is better estimated according to the Berlin definition of acute respiratory distress syndrome at standard 5 cmH2O rather than higher positive end-expiratory pressure: a retrospective cohort study. Crit Care Med 2015;43:781-90. [Crossref] [PubMed]
- De Luca D, Piastra M, Chidini G, et al. The use of the Berlin definition for acute respiratory distress syndrome during infancy and early childhood: multicenter evaluation and expert consensus. Intensive Care Med 2013;39:2083-91. [Crossref] [PubMed]
- Barreira ER, Munoz GO, Cavalheiro PO, et al. Epidemiology and outcomes of acute respiratory distress syndrome in children according to the Berlin definition: a multicenter prospective study. Crit Care Med 2015;43:947-53. [Crossref] [PubMed]
- Villar J, Perez-Mendez L, Lopez J, et al. An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2007;176:795-804. [Crossref] [PubMed]
- Ferguson ND, Kacmarek RM, Chiche JD, et al. Screening of ARDS patients using standardized ventilator settings: influence on enrollment in a clinical trial. Intensive Care Med 2004;30:1111-6. [Crossref] [PubMed]
- Britos M, Smoot E, Liu KD, et al. The value of positive end-expiratory pressure and Fio(2) criteria in the definition of the acute respiratory distress syndrome. Crit Care Med 2011;39:2025-30. [Crossref] [PubMed]
- Aboab J, Louis B, Jonson B, et al. Relation between PaO2/FIO2 ratio and FIO2: a mathematical description. Intensive Care Med 2006;32:1494-7. [Crossref] [PubMed]
- Rubenfeld GD, Caldwell E, Granton J, et al. Interobserver variability in applying a radiographic definition for ARDS. Chest 1999;116:1347-53. [Crossref] [PubMed]
- Meade MO, Cook RJ, Guyatt GH, et al. Interobserver variation in interpreting chest radiographs for the diagnosis of acute respiratory distress syndrome. Am J Respir Crit Care Med 2000;161:85-90. [Crossref] [PubMed]
- Lichtenstein D, Goldstein I, Mourgeon E, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004;100:9-15. [Crossref] [PubMed]
- Figueroa-Casas JB, Brunner N, Dwivedi AK, et al. Accuracy of the chest radiograph to identify bilateral pulmonary infiltrates consistent with the diagnosis of acute respiratory distress syndrome using computed tomography as reference standard. J Crit Care 2013;28:352-7. [Crossref] [PubMed]
- Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008;134:117-25. [Crossref] [PubMed]
- Leblanc D, Bouvet C, Degiovanni F, et al. Early lung ultrasonography predicts the occurrence of acute respiratory distress syndrome in blunt trauma patients. Intensive Care Med 2014;40:1468-74. [Crossref] [PubMed]
- Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound 2008;6:16. [Crossref] [PubMed]
- Cressoni M, Chiumello D, Algieri I, et al. Opening pressures and atelectrauma in acute respiratory distress syndrome. Intensive Care Med 2017;43:603-11. [Crossref] [PubMed]
- Chiumello D, Langer T, Vecchi V, et al. Low-dose chest computed tomography for quantitative and visual anatomical analysis in patients with acute respiratory distress syndrome. Intensive Care Med 2014;40:691-9. [Crossref] [PubMed]
- Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007;132:410-7. [Crossref] [PubMed]
- Bass CM, Sajed DR, Adedipe AA, et al. Pulmonary ultrasound and pulse oximetry versus chest radiography and arterial blood gas analysis for the diagnosis of acute respiratory distress syndrome: a pilot study. Crit Care 2015;19:282. [Crossref] [PubMed]
- Riviello ED, Kiviri W, Twagirumugabe T, et al. Hospital Incidence and Outcomes of the Acute Respiratory Distress Syndrome Using the Kigali Modification of the Berlin Definition. Am J Respir Crit Care Med 2016;193:52-9. [Crossref] [PubMed]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [Crossref] [PubMed]
- Pelosi P, D'Onofrio D, Chiumello D, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl 2003;42:48s-56s. [Crossref] [PubMed]
- Agarwal R, Srinivas R, Nath A, et al. Is the mortality higher in the pulmonary vs the extrapulmonary ARDS? A meta analysis. Chest 2008;133:1463-73. [Crossref] [PubMed]
- Cochi SE, Kempker JA, Annangi S, et al. Mortality Trends of Acute Respiratory Distress Syndrome in the United States from 1999 to 2013. Ann Am Thorac Soc 2016;13:1742-51. [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Zhi G, Xin W, Ying W, et al. "Obesity Paradox" in Acute Respiratory Distress Syndrome: Asystematic Review and Meta-Analysis. PLoS One 2016;11:e0163677. [Crossref] [PubMed]
- Ni YN, Luo J, Yu H, et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care 2017;21:36. [Crossref] [PubMed]
- Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest 2007;131:554-62. [Crossref] [PubMed]
- Moss M, Guidot DM, Steinberg KP, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med 2000;28:2187-92. [Crossref] [PubMed]
- McCallister JW, Adkins EJ, O'Brien JM Jr. Obesity and acute lung injury. Clin Chest Med 2009;30:495-508. viii. [Crossref] [PubMed]
- Shah D, Romero F, Guo Z, et al. Obesity-induced Endoplasmic Reticulum Stress Causes Lung Endothelial Dysfunction and Promotes Acute Lung Injury. Am J Respir Cell Mol Biol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 2003;31:869-77. [Crossref] [PubMed]
- Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003;97:1558-65. [Crossref] [PubMed]
- Yeligar SM, Chen MM, Kovacs EJ, et al. Alcohol and lung injury and immunity. Alcohol 2016;55:51-9. [Crossref] [PubMed]
- Simet SM, Sisson JH. Alcohol's Effects on Lung Health and Immunity. Alcohol Res 2015;37:199-208. [PubMed]
- Rosenberg AL, Dechert RE, Park PK, et al. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med 2009;24:35-46. [Crossref] [PubMed]
- Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564-75. [Crossref] [PubMed]
- Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med 2017;43:155-70. [Crossref] [PubMed]
- Manzano F, Yuste E, Colmenero M, et al. Incidence of acute respiratory distress syndrome and its relation to age. J Crit Care 2005;20:274-80. [Crossref] [PubMed]
- Erickson SE, Shlipak MG, Martin GS, et al. Racial and ethnic disparities in mortality from acute lung injury. Crit Care Med 2009;37:1-6. [Crossref] [PubMed]
- Meyer NJ, Christie JD. Genetic heterogeneity and risk of acute respiratory distress syndrome. Semin Respir Crit Care Med 2013;34:459-74. [Crossref] [PubMed]
- Ware LB, Zhao Z, Koyama T, et al. Long-Term Ozone Exposure Increases the Risk of Developing the Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2016;193:1143-50. [Crossref] [PubMed]
- Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med 2011;183:462-70. [Crossref] [PubMed]
- Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med 2002;30:1-6. [Crossref] [PubMed]
- Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. JAMA 2000;283:1995-2002. [Crossref] [PubMed]
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. [Crossref] [PubMed]
- Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2:611-20. [Crossref] [PubMed]
- Famous KR, Delucchi K, Ware LB, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med 2017;195:331-8. [PubMed]
- Tomashefski JF Jr. Pulmonary pathology of the adult respiratory distress syndrome. Clin Chest Med 1990;11:593-619. [PubMed]
- Albertine KH. Histopathology of pulmonary edema and the acute respiratory distress syndrome. In: Matthay MA, Ingbar DH. editors. Pulmonary Edema. New York: Marcel Dekker, 1998:37-83.
- Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982;3:35-56. [PubMed]
- Albertine KH. Ultrastructural abnormalities in increased-permeability pulmonary edema. Clin Chest Med 1985;6:345-69. [PubMed]
- de Hemptinne Q, Remmelink M, Brimioulle S, et al. ARDS: a clinicopathological confrontation. Chest 2009;135:944-9. [Crossref] [PubMed]
- Guerin C, Bayle F, Leray V, et al. Open lung biopsy in nonresolving ARDS frequently identifies diffuse alveolar damage regardless of the severity stage and may have implications for patient management. Intensive Care Med 2015;41:222-30. [Crossref] [PubMed]
- Cardinal-Fernández P, Bajwa EK, Dominguez-Calvo A, et al. The Presence of Diffuse Alveolar Damage on Open Lung Biopsy Is Associated With Mortality in Patients With Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Chest 2016;149:1155-64. [Crossref] [PubMed]
- Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012;122:2731-40. [Crossref] [PubMed]
- Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis 1977;116:589-615. [Crossref] [PubMed]
- Hoelz C, Negri EM, Lichtenfels AJ, et al. Morphometric differences in pulmonary lesions in primary and secondary ARDS. A preliminary study in autopsies. Pathol Res Pract 2001;197:521-30. [PubMed]
- Negri EM, Hoelz C, Barbas CS, et al. Acute remodeling of parenchyma in pulmonary and extrapulmonary ARDS. An autopsy study of collagen-elastic system fibers. Pathol Res Pract 2002;198:355-61. [Crossref] [PubMed]
- Pham T, Rubenfeld GD. Fifty Years of Research in ARDS. The Epidemiology of Acute Respiratory Distress Syndrome. A 50th Birthday Review. Am J Respir Crit Care Med 2017;195:860-70. [Crossref] [PubMed]
- Caser EB, Zandonade E, Pereira E, et al. Impact of distinct definitions of acute lung injury on its incidence and outcomes in Brazilian ICUs: prospective evaluation of 7,133 patients*. Crit Care Med 2014;42:574-82. [Crossref] [PubMed]
- Luhr OR, Antonsen K, Karlsson M, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med 1999;159:1849-61. [Crossref] [PubMed]
- Bersten AD, Edibam C, Hunt T, et al. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med 2002;165:443-8. [Crossref] [PubMed]
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685-93. [Crossref] [PubMed]
- Linko R, Okkonen M, Pettila V, et al. Acute respiratory failure in intensive care units. FINNALI: a prospective cohort study. Intensive Care Med 2009;35:1352-61. [Crossref] [PubMed]
- Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 2004;30:51-61. [Crossref] [PubMed]
- Sakr Y, Vincent JL, Reinhart K, et al. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest 2005;128:3098-108. [Crossref] [PubMed]
- Irish Critical Care Trials Group. Acute lung injury and the acute respiratory distress syndrome in Ireland: a prospective audit of epidemiology and management. Crit Care 2008;12:R30. [Crossref] [PubMed]
- Sigurdsson MI, Sigvaldason K, Gunnarsson TS, et al. Acute respiratory distress syndrome: nationwide changes in incidence, treatment and mortality over 23 years. Acta Anaesthesiol Scand 2013;57:37-45. [Crossref] [PubMed]
- Nolan S, Burgess K, Hopper L, et al. Acute respiratory distress syndrome in a community hospital ICU. Intensive Care Med 1997;23:530-8. [Crossref] [PubMed]
- Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med 2011;183:59-66. [Crossref] [PubMed]
- Villar J, Blanco J, Anon JM, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 2011;37:1932-41. [Crossref] [PubMed]
- Kneyber MC, Brouwers AG, Caris JA, et al. Acute respiratory distress syndrome: is it underrecognized in the pediatric intensive care unit? Intensive Care Med 2008;34:751-4. [Crossref] [PubMed]
- López-Fernández Y, Azagra AM, de la Oliva P, et al. Pediatric Acute Lung Injury Epidemiology and Natural History study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med 2012;40:3238-45. [Crossref] [PubMed]
- Erickson S, Schibler A, Numa A, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med 2007;8:317-23. [PubMed]
- Zimmerman JJ, Akhtar SR, Caldwell E, et al. Incidence and outcomes of pediatric acute lung injury. Pediatrics 2009;124:87-95. [Crossref] [PubMed]
- Adhikari NK, Fowler RA, Bhagwanjee S, et al. Critical care and the global burden of critical illness in adults. Lancet 2010;376:1339-46. [Crossref] [PubMed]
- Rubenfeld GD, Christie JD. The epidemiologist in the intensive care unit. Intensive Care Med 2004;30:4-6. [Crossref] [PubMed]
- Goss CH, Brower RG, Hudson LD, et al. Incidence of acute lung injury in the United States. Crit Care Med 2003;31:1607-11. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- McAuley DF, Laffey JG, O'Kane CM, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med 2014;371:1695-703. [Crossref] [PubMed]
- Laffey JG, Bellani G, Pham T, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med 2016;42:1865-76. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [Crossref] [PubMed]
- Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. [Crossref] [PubMed]
- Wong JJ, Jit M, Sultana R, et al. Mortality in Pediatric Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. J Intensive Care Med 2017.885066617705109. [PubMed]
- Schouten LR, Veltkamp F, Bos AP, et al. Incidence and Mortality of Acute Respiratory Distress Syndrome in Children: A Systematic Review and Meta-Analysis. Crit Care Med 2016;44:819-29. [PubMed]
- Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 2011;306:1574-81. [Crossref] [PubMed]
- Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011;184:561-8. [Crossref] [PubMed]
- Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med 2009;179:220-7. [Crossref] [PubMed]
- Erickson SE, Martin GS, Davis JL, et al. Recent trends in acute lung injury mortality: 1996-2005. Crit Care Med 2009;37:1574-9. [Crossref] [PubMed]


