Unusual case of spindle cell sarcoma metastases to right ventricle: a case report and a literature review
Introduction
A prevalence of 0.002–0.3% primary cardiac cancer is estimated in autopsy series. Cardiac metastases are 30 times more common than malignant primary tumors (1). Tumors that metastasize in the heart are cancers of lung (46%), breast (18%), uterus (9%), oesophagus (9%) and other neoplasms (18%). Primary cardiac sarcomas are more common than heart metastases from sarcoma (2).
Despite advances in local and systemic treatment of metastatic malignancies, due to their rarity there is no standard of care treatment for patients with cardiac metastases. The outcome is poor and most patients have widespread metastatic disease.
Palliative radiotherapy (RT) can play a significant role to control symptoms and prevent further cardiac function decline (3).
Here we present the case of a 30-year-old female with right ventricular metastases of spindle cell sarcoma of the sacral region with widespread disease.
Case presentation
In July 2011, a spindle cell sarcoma of the sacral region was diagnosed in a 27-year-old woman treated with systemic chemotherapy and proton therapy with complete response. Eighteen months later she showed one lung metastasis and therefore she underwent pulmonary wedge resection. Four months later new lung metastasis was diagnosed and treated with a further wedge resection and systemic chemotherapy.
Twelve months later she underwent stereotactic body radiotherapy (SBRT) on 4 new lung metastases (2 in the right and 2 in the left lung). The prescribed dose was 50 Gy in 5 fractions to 80% isodose.
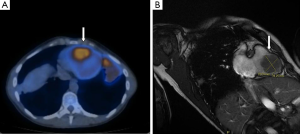
Four years after the initial diagnosis of sarcoma, she experienced dyspnea and general fatigue. Fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) showed widespread evidence of metabolically active metastatic disease in multiple pulmonary sites [standardized uptake value (SUV)max =7.4], right ileo-psoas muscle (SUVmax =6.7), soft tissue in the presacral region (SUVmax =7.2), left leg muscles (SUVmax =15.7), right arm brachial muscles (SUVmax =5.4) and right ventricle (SUVmax =1). Figure 1A shows the 18F-FDG uptake in the right ventricle at 18F-FDG PET/CT scan. Magnetic resonance (MR) imaging showed a 39.2 mm × 39.3 mm mass adherent to the interventricular septum with invasion of nearly all right ventricle cavity. The mass did not involve tricuspid valve or right atrial cavity. Figure 1B shows cardiac MR imaging of the right ventricular mass.
Although no biopsy was performed all lesions were considered as metastases from spindle cell sarcoma. Surgical resection was not recommended because of widespread disease and high surgical risk. The patient received oral anticoagulant therapy to prevent thromboembolism and was referred to our department for RT treatment.
Palliative RT was prescribed taking into account poor performance status, disease stage and previous SBRT to lung metastases. The delivered dose was 25 Gy in 5 fractions, prescribed to the 80% isodose.
Volumetric modulated arc technique was used. Image guided radiotherapy with cone beam CT was performed daily before each treatment fraction to check the position of the target volume. Online set-up adjustments were achieved using a robotic couch (HEXAPOD-EVO©; Elekta, Stockholm, Sweden). Patient immobilization was performed as previously reported (4). RT was well tolerated and no tumor size changes were observed during treatment.
One month after RT, no symptoms related to treatment were recorded [according to Radiation Therapy Oncology Group (5)] with complete resolution of dyspnea. Four months after RT she died because of extensive disseminated disease considered not amenable to any treatment.
Discussion
Cardiac metastases from sarcoma are rare. However, the incidence is increasing probably due to modern diagnostic tools and prolonged survival achieved by improved local and systemic therapies. Given the rarity of cardiac metastases no clinical data from randomized trials are available and therefore specific guidelines are lacking.
Factors like patient conditions (age, performance status, prognosis, and clinical symptoms), tumor characteristics (histology, size, location, and extent of systemic disease), and previous thoracic RT should be taken into account to define the treatment strategy. Surgical removal of cardiac metastases is usually taken into consideration in patients with better prognosis or in cases of intracardiac obstruction when complete or near-complete resection is possible (6).
However, most of patients have widespread metastatic disease with a very poor prognosis. RT can have a palliative role to relieve symptoms and prevent further cardiac function decline.
Here we present the case of an unusual cardiac metastasis from spindle cell sarcoma of sacral region. At the best to our knowledge, only one other case of cardiac metastases from spindle cell sarcoma was previously published (7). Agaimy and colleagues reported the case of a 38-year-old female with atrium-mitral valve metastases from spindle cell sarcoma of thoracic wall. Five months from treatment of primary sarcoma the patient developed one cardiac metastasis as the only site of relapse and therefore she underwent surgical resection. The patient died of cardiopulmonary failure 30 months later (7).
In our case surgery was not recommended because of widespread metastases and high surgical risk. She was symptomatic (dyspnea and general fatigue) and underwent palliative RT. An accelerated hypofractionated RT course was delivered (25 Gy total dose in 5 fractions, prescribed to the 80% isodose). One month after RT she was asymptomatic and 3 months later she died due to widespread disease not amenable to further treatments.
Again due to rarity of this metastases no study to define the optimal dose and fractionation is available. In the literature, only few reports on palliative RT in patients with cardiac metastases from sarcoma are available (8-10). Table 1 shows a summary of the papers reporting cardiac metastases from sarcoma treated with radiotherapy. Takenaka and colleagues retrospectively reported the largest case series (7 patients) of cardiac metastases from sarcoma treated with palliative RT. Dyspnea was the most frequent symptom and leiomyosarcoma (51%) was the most common histotype. All patients had other metastases. Different dose and fractionations were used (25 Gy/5 fractions, 45 Gy/15 fractions, 50 Gy/25 fractions, 60 Gy/30 fractions, 40 Gy/20 fractions, 32 Gy/16 fractions). Median survival of patients was 10.5 vs. 3.5 months recorded in 4 further patients not treated with RT (9). Ghiam and coworkers reported the results of 10 patients with cardiac metastases from different tumor histotypes treated with palliative RT. Two of 10 patients had cardiac metastases from sarcoma. Also in this paper dose and fraction were different: 50 and 45 Gy in 25 fractions. The response duration in these 2 patients was 11 and 7 months, respectively (8).
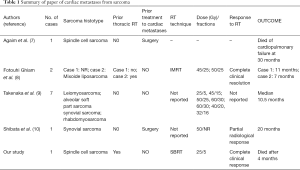
Full table
Shibata and coworkers reported 1 case of cardiac metastases from synovial sarcoma with partial radiological response after standard RT (50 Gy) (10).
Due to their rarity and poor outcome, cardiac metastases represent a challenging clinic problem. Treatment should be individualized in a multidisciplinary setting taking into account performance status, tumor characteristics and previous treatment, when possible surgery seems to be the best options. However, RT even in case of widespread disease can improve clinical control symptoms by reducing the mass effect.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol 1990;3:195-8. [PubMed]
- Hallahan DE, Vogelzang NJ, Borow KM, et al. Cardiac metastases from soft-tissue sarcomas. J Clin Oncol 1986;4:1662-9. [Crossref] [PubMed]
- Catton C. The management of malignant cardiac tumors: clinical considerations. Semin Diagn Pathol 2008;25:69-75. [Crossref] [PubMed]
- Frakulli R, Salvi F, Balestrini D, et al. Stereotactic Radiotherapy in the Treatment of Lung Metastases from Bone and Soft-tissue Sarcomas. Anticancer Res 2015;35:5581-6. [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341-6. [Crossref] [PubMed]
- Al-Mamgani A, Baartman L, Baaijens M, et al. Cardiac metastases. Int J Clin Oncol 2008;13:369-72. [Crossref] [PubMed]
- Agaimy A, Rösch J, Weyand M, et al. Primary and metastatic cardiac sarcomas: a 12-year experience at a German heart center. Int J Clin Exp Pathol 2012;5:928-38. [PubMed]
- Fotouhi Ghiam A, Dawson LA, Abuzeid W, et al. Role of palliative radiotherapy in the management of mural cardiac metastases: who, when and how to treat? A case series of 10 patients. Cancer Med 2016;5:989-96. [Crossref] [PubMed]
- Takenaka S, Hashimoto N, Araki N, et al. Eleven cases of cardiac metastases from soft-tissue sarcomas. Jpn J Clin Oncol 2011;41:514-8. [Crossref] [PubMed]
- Shibata T, Suehiro S, Hattori K, et al. Metastatic synovial sarcoma of the left ventricle. Jpn Heart J 2001;42:387-91. [Crossref] [PubMed]


