Transcatheter mitral valve replacement: still a long way to go!
Introduction
Catheter-based interventional procedures have become an increasingly offered treatment option for older and high-risk patients, especially in those contraindicated for surgical heart valve repair or replacement, because of frailty and/or comorbidities. Besides aortic valve pathologies, there is a broad spectrum of diseases (rheumatic, degenerative, ischemic and infectious) that may affect the mitral valve in a population that is increasingly aging.
Therefore, in addition to catheter-based repair techniques like artificial chords and mitra-clip as example, there is a need for transcatheter mitral valve replacement (TMVR) devices. Initial reports of success with transcatheter aortic valve replacement (TAVR) in previously placed annuloplasty rings and surgical prostheses confirmed the feasibility of treating MR with a valve-in-valve approach, prompting others to successfully implant TAVR prostheses in a native calcified mitral annulus (1-4).
However, compared to the aortic valve, the regurgitant mitral valve poses unique challenges for successful transcatheter valve deployment. The mitral valve has a more variable anatomy; it is noncircular, has a significantly larger orifice area than the aortic valve, it is dynamic in shape, usually noncalcified, and subject to cyclical, high left ventricular (LV) systolic pressures. In addition, the subvalvular apparatus is a complex structure to be included in the anchoring mechanism, and it is in close proximity to the LV outflow tract (LVOT). Finally the development of delivery catheters is demanding because of the more complex approach.
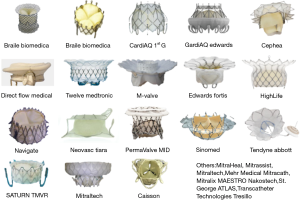
Interestingly, and in contrary with the very first experience of transcatheter valve implantation (where only two systems were available in the very first period), there are at least ten systems presently being assessed to obtain market acceptance (Figure 1), and another 20 systems in preclinical tests. This is most probably in direct relationship of the multiple encountered difficulties and the corresponding solutions tested. This particular condition makes the recruitment of patients for preclinical and clinical studies more difficult, because of the concurrential situation.
One of the most important criteria when patients are included in studies is a precise assessment of the size of the annulus. To accommodate this challenge, fusing imaging modalities seems to be the best solution: this means that different examinations, e.g., echocardiography (2D and 3D), CT-scan and/or MR imaging, are required to obtain the most precise evaluation. This was not the case so far, when pre-, intra- and post-operative evaluation of patients scheduled for mitral valve repair had to be performed (5).
Until now, there has been a few studies with TMVR and the results were by far less than optimal with mortality rate ranging roughly between 15% and 30%. Reasons for this high procedural mortality have been attributed to patient comorbidities, late treatment in natural history of the disease and operator learning curves.
However, it is more likely that these unpredictable outcomes were due to (I) inability to predict LVOT obstruction; (II) degree and distribution of calcifications of the mitral annulus and of the leaflets necessary for valve anchoring being unclear; (III) unknown algorithms for prosthesis sizing for seal in the shaped mitral annulus and finally (IV) foreshortening of the device in the left atrium being unpredictable (6-9).
Comment on the study by Muller and co-authors (JACC 2017)
The clinical trial published by Muller and co-authors in JACC in 2017, investigates a small series of 30 patients who received the Tendyne, self-expandable mitral valve prosthesis through a transapical approach (9). The feasibility design of the study required 30-day (or hospital) follow-up only. As in the majority of similar studies, exclusion criteria were strong, precluding therefore a larger number of patients to be considered. All exclusion criteria (e.g. LV end-diastolic diameter >70 mm, severe mitral annular or leaflet calcification, left atrial or LV thrombus, prior mitral or aortic valve surgery, prior transcatheter mitral intervention, pulmonary artery systolic pressure >70 mmHg, severe tricuspid regurgitation, and severe right ventricular dysfunction) are quite common clinical, anatomic or hemodynamic characteristics of so-called inoperable or high-risk patients scheduled for mitral valve surgery. As a result of the strong inclusion criteria, only 30 patients were recruited and treated in eight centers, with only three centers that treated more than two patients!
The Tendyne device is an apically tethered tri-leaflet porcine pericardial valve sewn onto a nitinol frame. It is specifically designed to address the complex mitral anatomy of functional, degenerative, and mixed etiology mitral regurgitation (10-11). One of the major advantage of the device system is the fact that if the function of the prosthesis is not acceptable or LVOT obstruction occurs, it can be recaptured and repositioned or fully retrieved, even after full deployment in the mitral annulus.
The Tendyne system has the following additional advantages. Firstly, the double frame design that allows adaptability to the asymmetric shape of the mitral valve annulus includes an outer frame with a cuff that rests against the anterior left atrial wall and the aorta. Secondly, the anchoring mechanism maintains stability and therefore minimizes the risk of prosthesis migration or embolization. Finally, the deployment ends with a secure closure of the apex that is facilitated by the application of the epicardial pad, that should minimize periprocedural bleeding.
In the study under discussion in this Editorial, thirty patients (mean age 75.6 years; 25 men) with grade 3 or 4 MR were selected. Etiology of the mitral regurgitation was secondary in 23, primary in 3, and mixed in 4 patients. The STS predicted risk of mortality was 7.3%±5.7%. Successful device implantation was achieved in 28 patients. There were no periprocedural death, stroke, or myocardial infarction. One patient died 13 days after TMVR from pneumonia. Leaflet thrombosis was detected in one patient at follow-up and resolved after increased oral anticoagulation with warfarin. At 30 days, transthoracic echocardiography showed mild central MR in one patient, and no residual MR in the remaining 26 patients with valves in situ. The LV end-diastolic volume index decreased from mean 90.1 mL/m2 at baseline to 72.1 mL/m2. Seventy-five percent of the patients reported mild or no symptoms at follow-up [New York Heart Association (NYHA) functional class I or II]. The authors concluded that TMVR is an effective and safe option for selected patients with symptomatic native MR.
It is self-explaining that further evaluation of TMVR is warranted. This intervention may help address an unmet need in patients at high risk for surgery, although the definition and interpretation of what is a “high-risk” or even “inoperable” patient is still a very critical issue. In the past, the definition of high-risk and inoperable patient frequently was confirmed by surgeons and/or anesthesiologists. Today, cardiologists themselves define more and more who is operable and who may not. This introduces a major bias in the decision process towards the procedure that the cardiologist may offer on his own.
In conclusion, this small series shows that Tendyne device implantation was successful in the majority of the patients. Primary performance endpoints were satisfactory and at 30 days, freedom from cardiovascular mortality, stroke, and device dysfunction was high. No information was available for a longer observational interval.
Comments
As recognized by the authors, there were several limitations related to this study: the number of highly-selected patients was small and the trial was not randomized. In additional, since optimization of medical therapy for heart failure was strongly recommended, it is not sure if the clinical benefits were due mainly because of the reduction of mitral regurgitation alone or if the medicamentous treatment also played a substantial role. The length of the follow-up was too short to investigate the risk of leaflet thrombosis, a phenomenon that has been described for both transcatheter and surgical valve therapies (12-14), but this may be even higher in the low flow velocity situation through the mitral valve.
Recently, other reports were published concerning TMVR. Altisent et al. published a small series on three patients suffering from functional mitral regurgitation with a severe reduction of LV function who received the Fortis TMVR device from Edwards under compassionate clinical use program because they were thought to be at very high risk for surgery (15). The procedure was performed through a transapical approach, and the valve was successfully implanted in the three patients. Echocardiography at discharge showed trace residual MR in two patients and no MR in one patient. At the 3-month follow-up, the valve function remained unchanged, and transesophageal echocardiography and computed tomography showed no structural failures. All patients had improvements in functional status, in exercise capacity as evaluated by 6-min walk test, and in quality of life. At 6-month follow-up, all patients remain alive with mild symptoms (NYHA functional class II) but without hospital readmission for heart failure.
While a strategy of mitral valve repair is usually preferred in cases of mitral regurgitation, the challenges in developing a viable mitral valve repair technology have led researchers to focus on valve replacement as a potentially simpler approach (16). Given the rapid evolution in device development, the complementary role of transcatheter mitral valve repair and replacement techniques must be considered speculative. In general, TMVR may be technically simpler and more reproducible in terms of MR reduction due to the principle “one valve fits all”. However, durability, safety and interaction with adjacent cardiac structures remain important concerns.
Even though there is no doubt that TMVR will do its way until final approvement by the cardiological and cardiosurgical community, a substantial number of questions have to be answered, about safety, effectiveness, user-friendly reproducibility, recapturability in case of inappropriate deployment and finally durability. To ensure a common language regarding the clinical outcome assessment, a mitral valve academic research consortium (VARC) has been created and has already published some statements regarding principles of clinical trial design and definition of endpoints. The multidisciplinary heart team, now established as a class I indication for the evaluation of complex patients with valvular heart disease, should play a central role in the use of this new technology.
The major advance for TMVR will be the computer-assisted simulation of valve insertion within the native mitral orifice. This will allow for proper pre-deployment assessment of the aorto-mitral angle, the LVOT-mitral valve angle and finally the estimation of the risk of LVOT obstruction.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Carrel T, Wenaweser P, Reineke S, et al. Worldwide first surgical implantation of a transcatheter valved stent in mitral position. Cardiovasc Med 2012;15:202-5. [Crossref]
- Cheung A, Webb JG, Barbanti M, et al. 5-year experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol 2013;61:1759-66. [Crossref] [PubMed]
- Lim ZY, Boix R, Prendergast B, et al. First reported case of transcatheter mitral valve implantation in mitral annular calcification with a fully repositionable and self-expanding valve. Circ Cardiovasc Interv 2015;8:e003031. [Crossref] [PubMed]
- Mellert F, Sinning JM, Werner N, et al. First-in-man transapical mitral valve replacement using the Direct Flow Medical® aortic valve prosthesis. Eur Heart J 2015;36:2119. [Crossref] [PubMed]
- Thériault-Lauzier P, Mylotte D, Dorfmeister M, et al. Quantitative multi-slice computed tomography assessment of the mitral valvular complex for transcatheter mitral valve interventions part 1: systematic measurement methodology and inter-observer variability. EuroIntervention 2016;12:e1011-20. [Crossref] [PubMed]
- Guerrero M, Dvir D, Himbert D, et al. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: results from the first multicenter global registry. JACC Cardiovasc Interv 2016;9:1361-71. [Crossref] [PubMed]
- Hermann H. CardiAQ-Edwards TMVR: design highlights and clinical update. Transcatheter Valve Therapies; 2016 June 16-18; Chicago, IL, USA.
- Rodés-Cabau J. FORTIS: design highlights and clinical update. Transcatheter Valve Therapies; 2016 June 16-18; Chicago, IL, USA.
- Verheye S, Cheung A, Leon M, et al. The Tiara transcatheter mitral valve implantation system. EuroIntervention 2015;11 Suppl W:W71-2.
- Muller DW, Farivar RS, Jansz P, et al. Transcatheter Mitral Valve Replacement for Patients With Symptomatic Mitral Regurgitation: A Global Feasibility Trial. J Am Coll Cardiol 2017;69:381-91. [Crossref] [PubMed]
- Moat N. Tendyne: design features and clinical update. Transcatheter Valve Therapies; 2016 June 16-18; Chicago, IL, USA.
- De Marchena E, Mesa J, Pomenti S, et al. Thrombus formation following transcatheter aortic valve replacement. JACC Cardiovasc Interv 2015;8:728-39. [Crossref] [PubMed]
- Butnaru A, Shaheen J, Tzivoni D, et al. Diagnosis and treatment of early bioprosthetic malfunction in the mitral valve position due to thrombus formation. Am J Cardiol 2013;112:1439-44. [Crossref] [PubMed]
- Hudec V, Bena M, Artemiou P, et al. Reversible thrombotic mitral valve stenosis after transcatheter mitral valve replacement (TMVR): Is life-long anticoagulation therapy necessary? J Card Surg 2017;32:190-2. [Crossref] [PubMed]
- Abdul-Jawad Altisent O, Dumont E, Dagenais F, et al. Initial Experience of Transcatheter Mitral Valve Replacement With a Novel Transcatheter Mitral Valve: Procedural and 6-Month Follow-Up Results. J Am Coll Cardiol 2015;66:1011-9. [Crossref] [PubMed]
- Jeevan RR, Murari BM. Engineering challenges and the future prospects of transcatheter mitral valve replacement technologies: a comprehensive review of case studies. Expert Rev Med Devices 2017;14:297-307. [Crossref] [PubMed]


