Abdominal aortic aneurysm: pictorial review of common appearances and complications
Introduction
Abdominal aortic aneurysms (AAA) are defined as abnormal dilatation of the abdominal aorta up to more than 3 cm in the greatest diameter or dilatation of more than 50% of its diameter. It remains a leading cause of death in the United States, nearing just less than 5,000 deaths due to the most dreaded complication, rupture (1). The risk factors include male gender, age >75 years, prior vascular disease, hypertension, cigarette smoking, family history, hypercholesterolemia (2). The most common location includes infrarenal with extension into iliac arteries. Atherosclerosis is the most common causative factor. Inflammatory abdominal aortic aneurysm, vasculitis such as Takayasu arteritis, connective tissue disorders such as Marfan syndrome and Ehlers-Danlos syndrome, mycotic aneurysm, traumatic pseudoaneurysm, anastomotic pseudoaneurysm are other notable causes. Treatment options include conservative follow up for aneurysms less than 5 cm to surgical open repair with graft placement as well as endovascular repair using stent graft placement. Overall, AAA accounts for approximately 45,000 operations in United States each year (1).
Clinical presentation
Most AAAs are asymptomatic and often incidentally detected. Unruptured aneurysms may uncommonly cause abdominal or back pain, or a pulsatile mass, if large. Ruptured aneurysms present with severe abdominal or back pain, hypotension and shock. Ruptured aneurysms are associated with high mortality (59–83% of patients succumb to death before they make it to hospital or undergo surgery).
Role of imaging
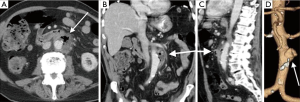
Imaging is essential for the detection of AAA, monitoring of rate of growth, pre-operative planning and post-operative follow-up. Although abdominal radiographs may show curvilinear calcification in the paravertebral region (Figure 1A), it is not optimal for detection or follow-up. Ultrasound is simple, safe and inexpensive with sensitivity of 95% and specificity close to 100%. It is the preferred modality of choice for monitoring of small aneurysms, especially in thin subjects.
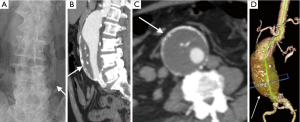
CTA is the gold standard and imaging modality of choice (3,4), but has high radiation doses. It has an excellent value for pre-operative planning including the evaluation of aortic size, rostral-caudal extent, presence of intraluminal thrombus, involvement of visceral arteries, extension into the suprarenal aorta (Figure 1B-D). It is superior to US in detecting and sizing common iliac artery aneurysms. MR angiography can provide the same information as CTA, but can be costlier, and less widely available and has longer imaging times. But this is especially useful in young patients due to lack of ionizing radiations and in situations where intravenous contrast is contraindicated, such as allergic reactions to intravenous contrast and renal failure (5). Role of digital subtraction angiography (DSA) is limited in the diagnosis of AAA due to advent of CTA. DSA also does not show true aneurysm size if there is mural thrombus. DSA is still an important tool in the endovascular treatment of the AAA (6).
Complications
The complications of AAA include rupture, infection, aorto-enteric fistula, aorto-caval fistulas pseudoaneurysm, thrombotic occlusion of branch vessel and compression of adjacent structures.
Rupture
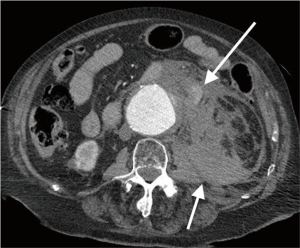
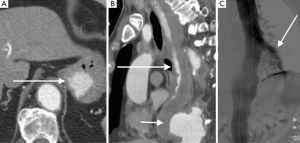
Acute rupture of AAA is a surgical emergency, and if left untreated, it has a mortality rate approaching 100%. The rupture most commonly involves the posterolateral aspect of the aortic wall, which results in hemorrhage into the retroperitoneal spaces including the perirenal space, pararenal spaces, and psoas muscles. Thus, the most common imaging feature of aneurysmal rupture is the presence of a retroperitoneal hematoma adjacent to AAA and peri-aortic stranding (Figure 2). Extravasation of IV contrast (Figure 3) reflects active bleeding (4). Secondary signs of rupture include high attenuation crescent (Figure 2), hematoma within either the mural thrombus or the aneurysmal wall, focal discontinuity of intimal calcification (Figure 2), tangential calcium sign, intimal calcification pointing away from the aneurysm, draped aorta sign, indistinct posterior aortic wall, posterior aorta following the contour of the spine on one or both sides (7).
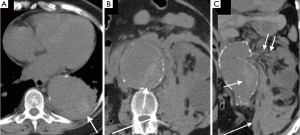
Contained rupture
The diagnostic criteria for contained rupture include known abdominal aortic aneurysm, previous pain symptoms that may have resolved, stable hemodynamic status with a normal hematocrit. CT imaging with show retroperitoneal hemorrhage, no evidence of active extravasation (Figure 4), with draping aorta sign (8).
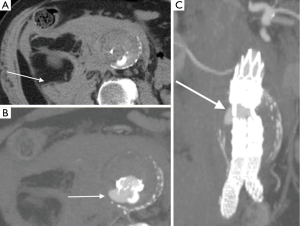
Infected (mycotic) aneurysms
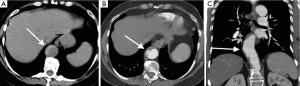
Mycotic aneurysms account for 0.7–2.6% of aortic aneurysms. There are very prone to rupture, with rupture rate of 53–75% at surgical repair. They can be caused by hematogenous seeding from septicemia or direct spread from vertebral osteomyelitis, renal and psoas abscesses (9). The majority are located in thoracic or suprarenal abdominal aorta. CT angiography will show saccular shape as opposed to fusiform shape in atherosclerotic aneurysms, lobular contours, and periaortic inflammation, abscess, and mass (Figure 5). They have rapid expansion rate than that of atherosclerotic aneurysms (10,11).
Aorto-enteric fistulas
Aorto-enteric fistulas can be primary, caused by atherosclerotic aortic aneurysm or secondary related to from aortic reconstructive surgery. Secondary aorto-enteric fistulas are more common than primary fistulas. They usually occur between two weeks and eight years after surgery. The most commonly involved part of the gut 3rd & 4th portions the duodenum. Symptoms usually include abdominal pain, hematemesis, and melena. CT imaging will show rupture with gas within or outside the aneurysm sac. However, this finding can be seen in mycotic aneurysms as well. The contrast extravasation into the bowel (Figure 6) is diagnostic (12).
Aorto-caval fistulas
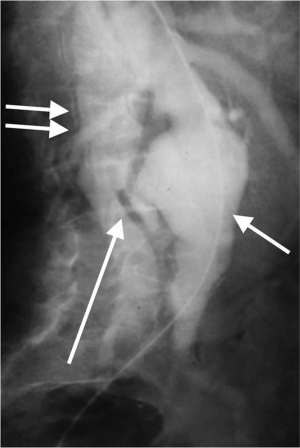
Aorto-caval fistula is a rare and devastating complication in which aneurysm erodes into the IVC. It accounts for <1% of all aneurysms and in ~3% of ruptured aortic aneurysms. The features can be very atypical leading to a delay in diagnosis. The clinical features include faintness, syncope, hypotension, high output cardiac failure, bilateral pedal edema, renal insufficiency (reduced renal blood flow) and continuous bruit. Imaging studies will show opacification of the IVC in the arterial phase (Figure 7). These patients need urgent surgical exploration with 20–55% operative mortality. The increased mortality is predominantly due to misdiagnosis or delayed diagnosis (13).
Pitfalls
On unenhanced CT, false lumen thrombus in aortic dissection and intramural hematoma of the abdominal aorta may mimic the hyperattenuating crescent sign, mimicking impending rupture in AAA (Figure 8). Furthermore, decreased attenuation of the patent lumen in patients with severe anemia due to low hematocrit level may also cause the arterial wall to appear hyperattenuated on unenhanced CT, thus simulating the hyperattenuating crescent sign. As a result, familiarity with such common pitfalls may be essential to improve diagnostic accuracy (14).
Treatment and prognosis
The risk of AAA rupture is proportional to the size of the aneurysm and the rate of growth (15). Rupture of AAA usually have devastating consequences, thus necessitating regular follow-up and urgent treatment. The treatment is usually required when diameter of AAA >5.0 cm in women, >5.5 to 6.0 cm in men, expansion rate >10 mm/yr. for any size and symptomatic patients. Management options include endovascular aneurysm repair (EVAR) and Open surgical repair (16,17).
Conclusions
Most patients with AAA are asymptomatic and the diagnosis is made incidentally. Imaging findings of aortic aneurysm rupture vary along a spectrum from impending rupture to contained rupture and from small aortic leaks with subtle infiltration of retroperitoneal fat to frank retroperitoneal or intraperitoneal extravasation. Aortic aneurysms most commonly occur as a consequence of atherosclerotic disease of the aorta. Alternatively, they may be associated with infectious seeding of the native or surgically repaired vessel (17). Prompt detection of abdominal aortic aneurysm rupture is critical because survival is improved by emergent surgery. Identification of impending or contained rupture is equally important because these patients are at risk for frank rupture, but can generally benefit from a more thorough preoperative assessment, followed by urgent surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- McPhee JT, Hill JS, Eslami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg 2007;45:891-9. [Crossref] [PubMed]
- Pande RL, Beckman JA. Abdominal aortic aneurysm: populations at risk and how to screen. J Vasc Interv Radiol 2008;19:S2-8. [Crossref] [PubMed]
- Wadgaonkar AD, Black JH 3rd, Weihe EK, et al. Abdominal Aortic Aneurysms Revisited: MDCT with Multiplanar Reconstructions for Identifying Indicators of Instability in the Pre- and Postoperative Patient. Radiographics 2015;35:254-68. [Crossref] [PubMed]
- Rakita D, Newatia A, Hines JJ, et al. Spectrum of CT findings in rupture and impending rupture of abdominal aortic aneurysms. Radiographics 2007;27:497-507. [Crossref] [PubMed]
- Rudarakanchana N, Powell JT. Advances in imaging and surveillance of AAA: when, how, how often? Prog Cardiovasc Dis 2013;56:7-12. [Crossref] [PubMed]
- Diehm N, Herrmann P, Dinkel HP. Multidetector CT angiography versus digital subtraction angiography for aortoiliac length measurements prior to endovascular AAA repair. J Endovasc Ther 2004;11:527-34. [Crossref] [PubMed]
- Schwartz SA, Taljanovic MS, Smyth S, et al. CT findings of rupture, impending rupture, and contained rupture of abdominal aortic aneurysms. AJR Am J Roentgenol 2007;188:W57-62. [Crossref] [PubMed]
- Halliday KE, al-Kutoubi A. Draped aorta: CT sign of contained leak of aortic aneurysms. Radiology 1996;199:41-3. [Crossref] [PubMed]
- Learch TJ, Sakamoto B, Ling AC, et al. Salmonella spondylodiscitis associated with a mycotic abdominal aortic aneurysm and paravertebral abscess. Emerg Radiol 2009;16:147-50. [Crossref] [PubMed]
- Lai CH, Chang RS, Luo CY, et al. Mycotic aneurysms in the abdominal aorta and iliac arteries: CT-based grading and correlation with surgical outcomes. World J Surg 2013;37:671-9. [Crossref] [PubMed]
- Azizi L, Henon A, Belkacem A, et al. Infected aortic aneurysms: CT features. Abdom Imaging 2004;29:716-20. [Crossref] [PubMed]
- Vu QD, Menias CO, Bhalla S, et al. Aortoenteric fistulas: CT features and potential mimics. Radiographics 2009;29:197-209. [Crossref] [PubMed]
- Lorenzati B, Perotto M, Bottone S, et al. Aortocaval fistula. Intern Emerg Med 2014;9:895-6. [Crossref] [PubMed]
- Morita S, Ueno E, Masukawa A, et al. Hyperattenuating sign at unenhanced CT indicating acute vascular disease. Radiographics 2010;30:111-25. [Crossref] [PubMed]
- Schermerhorn ML, Buck DB, O'Malley AJ, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015;373:328-38. [Crossref] [PubMed]
- Coscas R, Maumias T, Capdevila C, et al. Mini-invasive treatment of abdominal aortic aneurysms: current roles of endovascular, laparoscopic, and open techniques. Ann Vasc Surg 2014;28:123-31. [Crossref] [PubMed]
- Chauhan U, Puri SK, Jain N, et al. Percutaneous thrombin injection under sonographic guidance for exclusion of non-catheterizable post-pancreatitis pseudoaneurysm of the superior mesenteric artery: a minimally invasive and expeditious treatment option. J Med Ultrason (2001) 2016;43:295-9. [Crossref] [PubMed]


