Capsule retention: prevention, diagnosis and management
Introduction
Capsule endoscopy (CE) allows direct and non-invasive examination of the entire length of the small bowel and it has become the gold standard in evaluating suspected diseases of the small bowel (1-3). CE is considered an overall safe procedure; capsule retention (CR) remains the most relevant procedure-related complication, since it can potentially lead to acute SB obstruction and/or surgical intervention.
CR has been traditionally defined as having a capsule retained in the digestive tract for a minimum of 2 weeks (4). This definition has some limitations. First, the 2-week timeframe was arbitrarily selected by experts’ consensus. Second, it does not take into account either the CE findings or the patient’s symptoms. Nevertheless, this definition is so practical and easy to use that it is nowadays widely applied in both clinical practice and research setting.
Preventing CR
According to the abovementioned definition, in both large population-based studies and in a recently published meta-analysis of 67 studies, the overall CR rate is approximately 2% (5-13). Recently, Iijima et al. (14) evaluated the time-trend of CR rate, by comparing US, EU and Japan pre-marketing and post-marketing data. Interestingly, the authors found that CR rate has remained stable in over 10 years.
Several studies have been performed to identify risk factors associated with CR. CR rate does not correlate with capsule size (13-17), and/or patient’s age (18-20). Conversely, the rate strictly depends on clinical indication. When CE was performed in 773 young healthy volunteers during a pharmaceutical trial, no CR was reported (21). The retention rate in patients with suspected small bowel bleeding is approximately 2% (range: 0–7%), which is similar to that observed in patients undergoing CE for chronic diarrhea or abdominal pain (6). However, it increases up to 13% (22) in patients with a higher risk of harboring small bowel strictures, such as those with inflammatory bowel disease (IBD). Among them, a substantially higher CR rate is reported in case of established versus suspected IBD (8.0% versus 3.5%) (6). The highest CR rate has been observed in patients undergoing CE for subacute small bowel obstruction (10–20%) (23,24) or in patients with small bowel tumors (10–25%) (25-27). Furthermore, previous small bowel resection, abdominal radiation therapy and chronic use of high-dose non-steroidal anti-inflammatory drugs (NSAIDs) (9,28-31) are considered risk factors for CR. All these data emphasize the need to carefully assess the past clinical history of patients, before performing CE, in order to identify those deserving a dedicated preliminary work-up aimed at preventing CR.
For this purpose, both small bowel follow-trough (SBFT) and abdominal computed tomography (CT) have been demonstrated to be highly inaccurate, with a majority of patients with CR having a previous normal examination (7,32,33). Two other modalities have been more recently proposed, in order to prevent CR: Patency capsule® (PC) and dedicated small bowel cross-sectional imaging techniques, including CT enterography/enteroclysis (CTE) and magnetic resonance enterography/enteroclysis (MRE).
The PC system is described in detail elsewhere (34). Since its use closely mimics a CE procedure, it would be the ideal tool, at least form a theoretical point of view, to test small bowel patency before CE. Furthermore, it does not necessarily require exposure to radiation, it is relatively inexpensive and it can be easily repeated. Fernández-Urién et al. (7) retrospectively checked more than 5,400 CE procedures performed in over ten years (from 2001 to 2012) and found that, although no significant differences were disclosed, the CR rate was higher in pre-PC era and in those institutions that had never used PC, than in post-PC era: 1.7% and 1.8% versus 1.2%, respectively. Consistently, several case series (33-38) and a recently published meta-analysis (39) (overall including five studies and 203 patients) confirmed the accuracy of PC test, with a sensitivity of 97% (95% CI, 93–99%), a specificity of 83% (95% CI, 65–94%) and an area under the receiving operator curve (ROC) of 0.9557. However, although a negative PC test (i.e., when PC is excreted intact or the radiofrequency scanner does not identify the tag within the predefined time of dissolution) seems to effectively minimize the risk of CR (40-42), the specificity of the PC test remains an issue. In fact, Nemeth et al. (43) reported that among 18 IBD patients with positive PC test who eventually underwent CE, only two experienced CR. Other authors (40) documented no case of retention in 5 patients with spondyloarthritis, despite a positive PC test (i.e., capsule documented in patient’s body after the predefined time of dissolution, or caspsule excreted in fragments within the predefined time of dissolution). Therefore, these data seem to question the discriminative power of PC, since all patients with positive PC are excluded from CE, although this examination might be helpful in the diagnostic process. Moreover, some patients, referred to PC to prevent CR and hence a potential acute obstruction, developed obstructive symptoms induced by the PC (44-47).
As far as dedicated small bowel cross-sectional techniques are concerned, they have the advantage to be widely available, to be reimbursed, and especially to provide a comprehensive staging of the underlying disease thanks to their panoramic view. Nevertheless, although some differences between CT- and MR-based techniques exist, their overall sensitivity in identifying small bowel strictures remains largely suboptimal (41,48,49) and, even when a tight stenosis is identified, this does not reliably predict the capsule passage (50). Last but not least, the reliability of dedicated cross-sectional imaging techniques appears to be highly operator-dependent (51). This is a relevant issue, since it can impact on their use in the everyday clinical practice.
Unfortunately, studies comparing PC and dedicated small bowel cross-sectional imaging techniques in patients at increased risk for CR are limited, at present time. Particularly, there are no back-to-back or randomized studies, and available retrospective studies or case series yielded conflicting results. In the study by Yadav et al. (42) the authors showed a substantial equivalence between the two techniques (negative predictive value of 91% and 94% for PC and radiological techniques, respectively). However, this study was carried out in a single tertiary referral center with dedicated radiologists and highly selected patients (42 patients, 60% of them with Crohn’s disease). Conversely, a multicenter Italian study (41), carried out in a clinical practice setting, including a large number of centers and unselected patients with different risk factors for CR, showed that the retention rate was significantly lower (0.7%) in high risk patients with negative PC than in those with negative dedicated small bowel cross-sectional imaging (8.3%). In the light of these limited data, we can conclude that cross-sectional techniques and PC are both effective in decreasing the retention rate, although none of them is able to completely eliminate the risk of retention. Interestingly, as showed by Yadav et al. (42), the sensitivity in predicting CR can reach 100%, when PC and dedicated small bowel cross-sectional imaging techniques are combined. This could be the starting point for planning new protocols in the future, in which the combination of different methods will allow to offset present limitations, making CE even safer.
CR diagnosis
Promptly suspecting and quickly undertaking the necessary work-up to confirm CR are of paramount importance. In fact, the diagnosis of CR has relevant clinical implications (1). Although CR is usually asymptomatic, some cases of acute obstruction and perforation have been reported (6-13,52-56). Furthermore, if CR is readily diagnosed, some subgroups of patients can receive medical treatments (i.e., steroids in IBD patients) (7,57,58), which may favor capsule excretion in up to 20–30% of cases (7,57,58). Last but not least, some patients having performed CE might need to undergo MR imaging tests in the future. Although two case reports described that MR was uneventfully performed in capsule carriers (59,60), it is still contraindicated and potentially harmful in these patients.
CR should be suspected in two groups of patients: (I) asymptomatic patients who have not reported capsule excretion within 15 days after its ingestion; and (II) those developing obstructive or perforation-related symptoms in which the capsule has not certainly been excreted. Interestingly, in the second group of patients, the index of suspicion must remain high regardless of the time elapsed between capsule ingestion and the onset of symptoms, as several cases in which acute obstruction has occurred several years after capsule ingestion are reported in the literature (54,61,62). Fortunately, patients with symptomatic CR are a small minority (less than 2% of all retentions) (7,8,10-12). Because of clinical symptoms, these patients first undergo abdominal CT and eventually surgical intervention, which is mainly aimed at treating the cause of CR, along with capsule retrieval. The first group of patients represents the large majority of patients with suspected CR. Although all patients undergoing CE receive detailed oral and written instructions about the need to carefully check the feces to ensure capsule excretion (2), many of them do not give the necessary importance to this task (5), mostly because of reluctance in checking their feces. In fact, some cases in which the retention was accidentally discovered several years after capsule ingestion have been reported (52,53,63).
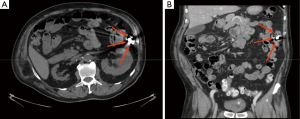
Nowadays, in asymptomatic patients, plain abdominal X-ray, performed 15 days after capsule ingestion, is the preferred tool to confirm CR. This test is readily available worldwide, easy to perform, non-invasive, repeatable and inexpensive. Furthermore, thanks to the presence of metal internal components (i.e., the batteries), the capsule is easy to identify by X-ray. Rarely, metallic implants (e.g., vascular stents, staples) might mask the capsule and, whenever it is reduced in small fragments, these can be difficult to detect. In addition, the determination of the exact location of a retained capsule (i.e., distinguishing between small or large bowel) by plain abdominal X-ray may be challenging. To overcome these drawbacks, the use of CT has been therefore suggested (54,56,64). CT can give a precise capsule location (Figure 1) and provide data about the cause of CR, however it implies the exposure to a higher dose of ionizing radiations. Therefore, it should be used only in selected patients, when an accurate capsule location is needed (e.g., when planning capsule retrieval, etc.) or when clinically indicated (e.g., to evaluate possible cause of CR). Interestingly, Girelli et al. (65) have successfully identified a retained capsule by ultrasound, as it sonographically appears as a hyperechoic egg-shaped image with acoustic shadowing. Nevertheless, small bowel ultrasound requires a specific training and can provide only limited data about precise capsule location.
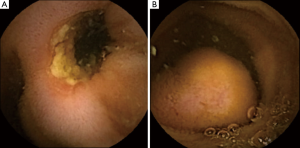
In asymptomatic patients, when CE findings (i.e., strictures or obstructing masses, Figure 2) suggest a possible CR, a timeframe shorter than two weeks for the X-ray is advisable. In these patients, the subsequent diagnostic work-up is mostly driven by capsule findings, but the evidence of CR may impact on the timing and selection of further diagnostic and therapeutic procedures. Nevertheless, there are no data about the optimal timing for performing the X-ray in these patients. Since in clinical practice the majority of capsules are excreted within 3–7 days (5), in order to avoid unnecessary X-ray exposure, it seems reasonable to plan an abdominal X-ray after 7 instead of 15 days after ingestion in these patients.
On the other hand, some authors (5) also suggest that performing X-ray might be completely avoided in asymptomatic patients with complete CE (i.e., capsule reaching the cecum during the recording time). Sachdev et al. (5) reported that in 75 of 115 patients, the capsule successfully reached the colon during recording time, and none of these patients had CR. Therefore, reaching the cecum during recording is considered reassuring because cases of colonic retention in patients undergoing CE for the evaluation of the small bowel are very rare (less than 1% of all retentions) (7).
Managing CR
As mentioned above, in patients with CR and obstructive or perforation-related symptoms, an individualized diagnostic work-up should be promptly undertaken. Conversely, an initial watchful monitoring is recommended (1) in asymptomatic patients with X-ray-proven CR.
This approach is advisable for several reasons. First, 35–50% of patients with CR will excrete capsule naturally after more than 15 days without any therapy (7,12). Furthermore, as already mentioned, medical therapy can facilitate capsule excretion in selected subgroups of patients (7,57,58). Last but not least, acute obstruction is rare. Nevertheless, although a spontaneous uneventful natural excretion occurring 2.5 years after capsule ingestion has been published (8), some case reports (29,54-56) described complications in patients with long-lasting CR. In these patients the retention remained asymptomatic for years, and unpredictably perforation or obstruction occurred. These complications seem to be due to capsule remaining for long time in pre-stenotic dilated segments or to capsule disintegration with potentially harmful fragments (i.e., small flat batteries) navigating within the small bowel lumen. Therefore, in asymptomatic patients, despite the initial watchful monitoring remains the most reasonable option, leaving the capsule un-retrieved for a long time can be harmful and may expose patients to delayed complications.
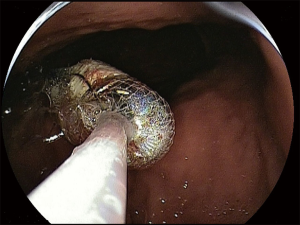
At present there are no studies exploring the optimal timing for capsule retrieval in asymptomatic patients. However, since spontaneous excretion, as well as the onset of acute obstructive symptoms, usually occurs in 4–12 weeks (7,8) after ingestion, capsule retrieval should be reasonably proposed if the capsule should be reasonably proposed in 3–6 months in these patients. At that time the patient has to be fully informed about both the risks of holding the capsule for longer and about the available options for capsule retrieval. These are basically represented by surgical intervention and endoscopy. In the early CE studies (9,32,66,67), almost all patients with CR were referred for surgical intervention. Nowadays, the advent of new endoscopic techniques, allowing for an extensive evaluation of the small bowel in a mini invasive way [namely device-assisted enteroscopy (DAE)] marginalized the need for surgery. Nonetheless, surgical intervention remains the first choice in all cases in which diagnostic tools unequivocally suggest the presence of a neoplastic disease. In these cases surgery is primarily aimed at treating the small bowel disease, simultaneously allowing the capsule retrieval. If capsule is retained in the small bowel and no surgical treatment is required, DAE has proven to be extremely effective (90–100% of cases) in ensuring the capsule retrieval (Figure 3) (68-71). Similarly, gastroscopy and push enteroscopy have been used to retrieve capsule retained in the upper gastrointestinal tract (e.g., in patients with gastric retention, Zenker’s or duodenal diverticula) (32,72,73).
Conclusions
CE has been introduced in clinical practice more than 15 years ago. The experience accumulated confirms that it is a safe examination and CR remains the only relevant procedure-related complication, whose frequency, as well as CR risk factors, has been clearly documented in very large population based studies in different Countries. Similarly, the tendency of CR to be asymptomatic for a long time is substantially confirmed in many case reports and case series, and studies describing the possibility to endoscopically retrieve the capsule are based on convincing and consistent case series. Conversely, the available studies on CR prevention are characterized by important methodological limitations, which make it difficult to draw firm conclusions as well as to translate them in everyday clinical practice. In this setting, large, prospective randomized or back-to-back studies are needed to determine which is the best preventive strategy.
Acknowledgements
I would like to thank Dr. Silvia Paggi and Dr. Franco Radaelli for their invaluable help in the editing process.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015;47:352-76. [Crossref] [PubMed]
- Koulaouzidis A, Rondonotti E, Karargyris A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J Gastroenterol 2013;19:3726-46. [Crossref] [PubMed]
- Gerson LB, Fidler JL, Cave DR, et al. ACG clinical guideline: diagnosis and management of small bowel bleeding. Am J Gastroenterol 2015;110:1265-87. [Crossref] [PubMed]
- Cave D, Legnani P, de Franchis R, et al. ICCE consensus for capsule retention. Endoscopy 2005;37:1065-7. [Crossref] [PubMed]
- Sachdev MS, Leighton JA, Fleischer DE, et al. A prospective study of the utility of abdominal radiographs after capsule endoscopy for the diagnosis of capsule retention. Gastrointest Endosc 2007;66:894-900. [Crossref] [PubMed]
- Rezapour M, Amadi C, Gerson L. Retention associated with video capsule endoscopy: systematic review and meta-analysis. Gastrointest Endosc 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Fernández-Urién I, Carretero C, González B, et al. Incidence, clinical outcomes, and therapeutic approaches of capsule endoscopy-related adverse events in a large study population. Rev Esp Enferm Dig 2015;107:745-52. [Crossref] [PubMed]
- Höög CM, Bark LÅ, Arkani J, et al. Capsule retentions and incomplete capsule endoscopy examinations: an analysis of 2300 examinations. Gastroenterol Res Pract 2012;2012:518718. [Crossref] [PubMed]
- Li F, Gurudu SR, De Petris G, et al. Retention of the capsule endoscope: a single-center experience of 1000 capsule endoscopy procedures. Gastrointest Endosc 2008;68:174-80. [Crossref] [PubMed]
- Liao Z, Gao R, Xu C, et al. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc 2010;71:280-6. [Crossref] [PubMed]
- Lim YJ, Lee OY, Jeen YT, et al. Indications for Detection, Completion, and Retention Rates of Small Bowel Capsule Endoscopy Based on the 10-Year Data from the Korean Capsule Endoscopy Registry. Clin Endosc 2015;48:399-404. [Crossref] [PubMed]
- Rondonotti E, Soncini M, Girelli C, et al. Small bowel capsule endoscopy in clinical practice: a multicenter 7-year survey. Eur J Gastroenterol Hepatol 2010;22:1380-6. [Crossref] [PubMed]
- Liao Z, Gao R, Li F, et al. Fields of applications, diagnostic yields and findings of OMOM capsule endoscopy in 2400 Chinese patients. World J Gastroenterol 2010;16:2669-76. [Crossref] [PubMed]
- Iijima K, Umezu M, Iwasaki K. Time series analysis of the effectiveness and safety of capsule endoscopy between the premarketing and postmarketing settings: a meta-analysis. PLoS One 2016;11:e0153662. [Crossref] [PubMed]
- Mussetto A, Fuccio L, Dari S, et al. MiroCam capsule for obscure gastrointestinal bleeding: a prospective, single centre experience. Dig Liver Dis 2013;45:124-8. [Crossref] [PubMed]
- Tontini GE, Wiedbrauck F, Cavallaro F, et al. Small-bowel capsule endoscopy with panoramic view: results of the first multicenter, observational study (with videos). Gastrointest Endosc 2017;85:401-8. [Crossref] [PubMed]
- Cave DR, Fleischer DE, Leighton JA, et al. A multicenter randomized comparison of the Endocapsule and the Pillcam SB. Gastrointest Endosc 2008;68:487-94. [Crossref] [PubMed]
- Atay O, Mahajan L, Kay M, et al. Risk of capsule endoscope retention in pediatric patients: a large single-center experience and review of the literature. J Pediatr Gastroenterol Nutr 2009;49:196-201. [Crossref] [PubMed]
- Friedlander JA, Liu QY, Sahn B, et al. NASPGHAN Capsule Endoscopy Clinical Report. J Pediatr Gastroenterol Nutr 2017;64:485-94. [Crossref] [PubMed]
- Gómez V, Cheesman AR, Heckman MG, et al. Safety of capsule endoscopy in the octogenarian as compared with younger patients. Gastrointest Endosc 2013;785:744-9. [Crossref] [PubMed]
- Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol 2005;3:133-41. [Crossref] [PubMed]
- Cheifetz AS, Lewis BS. Capsule endoscopy retention: is it a complication? J Clin Gastroenterol 2006;40:688-91. [Crossref] [PubMed]
- Cheifetz A, Sachar D, Lewis B. Small bowel obstruction: indication or contraindication for capsule endoscopy. Gastrointest Endosc 2004;59:102. [Crossref]
- Yang XY, Chen CX, Zhang BL, et al. Diagnostic effect of capsule endoscopy in 31 cases of subacute small bowel obstruction. World J Gastroenterol 2009;15:2401-5. [Crossref] [PubMed]
- Pasha SF, Sharma VK, Carey EJ et al. Utility of video capsule endoscopy in the detection of small bowel tumors. A single center experience of 1000 consecutive patients. Proceedings of the 6th International Conference on Capsule Endoscopy. 2007 June 8-10, Madrid, Spain, 2007:45.
- Bailey AA, Debinski HS, Appleyard MN, et al. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a threecenter Australian experience. Am J Gastroenterol 2006;101:2237-43. [Crossref] [PubMed]
- Rondonotti E, Pennazio M, Toth E, et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy 2008;40:488-95. [Crossref] [PubMed]
- Signorelli C, Rondonotti E, Villa F, et al. Use of the Given Patency System for the screening of patients at high risk for capsule retention. Dig Liver Dis 2006;38:326-30. [Crossref] [PubMed]
- Postgate AJ, Burling D, Gupta A, et al. Safety, reliability and limitations of the given patency capsule in patients at risk of capsule retention: a 3-year technical review. Dig Dis Sci 2008;53:2732-8. [Crossref] [PubMed]
- Al-Bawardy B, Locke G, Huprich JE, et al. Retained Capsule Endoscopy in a Large Tertiary Care Academic Practice and Radiologic Predictors of Retention. Inflamm Bowel Dis 2015;21:2158-64. [Crossref] [PubMed]
- Du J, Pan D, Ma P, et al. The clinical characteristic and risk of capsule incomplete and retention in Crohn's disease. Int J Clin Exp Med 2015;8:13482-90. [PubMed]
- Rondonotti E, Herrerias JM, Pennazio M, et al. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc 2005;62:712-6. [Crossref] [PubMed]
- Spada C, Spera G, Riccioni M, et al. A novel diagnostic tool for detecting functional patency of the small bowel: the Given patency capsule. Endoscopy 2005;37:793-800. [Crossref] [PubMed]
- Caunedo-Álvarez A, Romero-Vazquez J, Herrerias-Gutierrez JM. Patency and Agile capsules. World J Gastroenterol 2008;14:5269-73. [Crossref] [PubMed]
- Herrerias JM, Leighton JA, Costamagna G, et al. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc 2008;67:902-9. [Crossref] [PubMed]
- Nakamura M, Hirooka Y, Yamamura T, et al. Clinical usefulness of novel tag-less Agile patency capsule prior to capsule endoscopy for patients with suspected small bowel stenosis. Dig Endosc 2015;27:61-6. [Crossref] [PubMed]
- Delvaux M, Ben Soussan E, Laurent V, et al. Clinical evaluation of the use of the M2A patency capsule system before a capsule endoscopy procedure, in patients with known or suspected intestinal stenosis. Endoscopy 2005;37:801-7. [Crossref] [PubMed]
- Boivin ML, Lochs H, Voderholzer WA. Does passage of a patency capsule indicate small-bowel patency? A prospective clinical trial. Endoscopy 2005;37:808-15. [Crossref] [PubMed]
- Zhang W, Han ZL, Cheng Y, et al. Value of the patency capsule in pre-evaluation for capsule endoscopy in cases of intestinal obstruction. J Dig Dis 2014;15:345-51. [Crossref] [PubMed]
- Gheorghe A, Zahiu DC, Voiosu TA, et al. Is the use of AGILE patency capsule prior to videocapsule endoscopy useful in all patients with spondyloarthritis? Rom J Intern Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Rondonotti E, Soncini M, Girelli CM, et al. Short article: Negative small-bowel cross-sectional imaging does not exclude capsule retention in high-risk patients. Eur J Gastroenterol Hepatol 2016;28:871-5. [Crossref] [PubMed]
- Yadav A, Heigh RI, Hara AK, et al. Performance of the patency capsule compared with nonenteroclysis radiologic examinations in patients with known or suspected intestinal strictures. Gastrointest Endosc 2011;74:834-9. [Crossref] [PubMed]
- Nemeth A, Kopylov U, Koulaouzidis A, et al. Use of patency capsule in patients with established Crohn's disease. Endoscopy 2016;48:373-9. [PubMed]
- Kopylov U, Nemeth A, Cebrian A, et al. Symptomatic retention of the patency capsule: a multicenter real life case series. Endosc Int Open 2016;4:E964-9. [Crossref] [PubMed]
- Kato S, Osada H, Yakabi K. Rare case of temporary intestinal obstruction induced by novel tag-less Agile patency capsule in a patient with Crohn's disease. Dig Endosc 2016;28:481. [Crossref] [PubMed]
- Saito K, Nakagawa T, Koseki H, et al. Retention of the cellophane wall of a patency capsule by intestinal stenosis: a report of three cases. Clin J Gastroenterol 2016;9:365-8. [Crossref] [PubMed]
- Gay G, Delvaux M, Laurent V, et al. Temporary intestinal occlusion induced by a "patency capsule" in a patient with Crohn's disease. Endoscopy 2005;37:174-7. [Crossref] [PubMed]
- Ohtsuka K, Takenaka K, Kitazume Y, et al. Magnetic resonance enterography for the evaluation of the deep small intestine in Crohn's disease. Intest Res 2016;14:120-6. [Crossref] [PubMed]
- Fiorino G, Bonifacio C, Peyrin-Biroulet L, et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn's disease. Inflamm Bowel Dis 2011;17:1073-80. [Crossref] [PubMed]
- Spada C, Shah SK, Riccioni ME, et al. Video capsule endoscopy in patients with known or suspected small bowel stricture previously tested with the dissolving patency capsule. J Clin Gastroenterol 2007;41:576-82. [Crossref] [PubMed]
- Jensen MD, Ormstrup T, Vagn-Hansen C, et al. Interobserver and intermodality agreement for detection of small bowel Crohn's disease with MR enterography and CT enterography. Inflamm Bowel Dis 2011;17:1081-8. [Crossref] [PubMed]
- Saigusa S, Ohi M, Imaoka H, et al. Unawareness of a prolonged retained capsule endoscopy: the importance of careful follow-up and cooperation between medical institutions. Case Rep Gastrointest Med 2014;2014:909360. [Crossref] [PubMed]
- Fry LC, De Petris G, Swain JM, et al. Impaction and fracture of a video capsule in the small bowel requiring laparotomy for removal of the capsule fragments. Endoscopy 2005;37:674-6. [Crossref] [PubMed]
- De Palma GD, Masone S, Persico M, et al. Capsule impaction presenting as acute small bowel perforation: a case series. J Med Case Rep 2012;6:121. [Crossref] [PubMed]
- Rogers AM, Kuperman E, Puleo FJ, et al. Intestinal obstruction by capsule endoscopy in a patient with radiation enteritis. JSLS 2008;12:85-7. [PubMed]
- Royall NA, Fiscina CD. Report of video-capsule endoscopy disruption producing episodic small bowel obstruction after prolonged retention. Int J Surg Case Rep 2014;5:1001-4. [Crossref] [PubMed]
- Vanfleteren L, van der Schaar P, Goedhard J. Ileus related to wireless capsule retention in suspected Crohn's disease: emergency surgery obviated by early pharmacological treatment. Endoscopy 2009;41 Suppl 2:E134-5. [Crossref] [PubMed]
- Cheon JH, Kim YS, Lee IS, et al. Can we predict spontaneous capsule passage after retention? A nationwide study to evaluate the incidence and clinical outcomes of capsule retention. Endoscopy 2007;39:1046-52. [Crossref] [PubMed]
- Berry PA, Srirajaskanthan R, Anderson SH. An urgent call to the magnetic resonance scanner: potential dangers of capsule endoscopy. Clin Gastroenterol Hepatol 2010;8:A26. [Crossref] [PubMed]
- Anderson BW, Liang JJ, Dejesus RS. Capsule endoscopy device retention and magnetic resonance imaging. Proc (Bayl Univ Med Cent) 2013;26:270-1. [PubMed]
- Skovsen AP, Burcharth J, Burgdorf SK. Capsule endoscopy: a cause of late small bowel obstruction and perforation. Case Rep Surg 2013;2013:458108. [Crossref] [PubMed]
- Strosberg JR, Shibata D, Kvols LK. Intermittent bowel obstruction due to a retained wireless capsule endoscope in a patient with a small bowel carcinoid tumour. Can J Gastroenterol 2007;21:113-5. [Crossref] [PubMed]
- Araujo IK, Pages M, Romero C, et al. Twelve-year asymptomatic retention of a colon capsule endoscope. Gastrointest Endosc 2017;85:681-2. [Crossref] [PubMed]
- Bakhshi GD, Tayade MB, Jadhav KV, et al. Retention of an endoscopic capsule. J Minim Access Surg 2014;10:163-5. [Crossref] [PubMed]
- Girelli CM, Amato A, Rocca F. Easy ultrasound detection of retained video endoscopy capsule. J Gastroenterol Hepatol 2004;19:241. [Crossref] [PubMed]
- Cheifetz AS, Kornbluth AA, Legnani P, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol 2006;101:2218-22. [Crossref] [PubMed]
- Fry LC, Carey EJ, Shiff AD, et al. The yield of capsule endoscopy in patients with abdominal pain or diarrhea. Endoscopy 2006;38:498-502. [Crossref] [PubMed]
- Lee BI, Choi H, Choi KY, et al. Retrieval of a retained capsule endoscope by double-balloon enteroscopy. Gastrointest Endosc 2005;62:463-5. [Crossref] [PubMed]
- May A, Nachbar L, Ell C. Extraction of entrapped capsules from the small bowel by means of push-and-pull enteroscopy with the double-balloon technique. Endoscopy 2005;37:591-3. [Crossref] [PubMed]
- Van Weyenberg SJ, Van Turenhout ST, Bouma G, et al. Double-balloon endoscopy as the primary method for small bowel video capsule endoscope retrieval. Gastrointest Endosc 2010;71:535-41. [Crossref] [PubMed]
- Mitsui K, Fujimori S, Tanaka S, et al. Retrieval of Retained Capsule Endoscopy at Small Bowel Stricture by Double-Balloon Endoscopy Significantly Decreases Surgical Treatment. J Clin Gastroenterol 2016;50:141-6. [Crossref] [PubMed]
- Simmons DT, Baron TH. Endoscopic retrieval of a capsule endoscope from a Zenker's diverticulum. Dis Esophagus 2005;18:338-9. [Crossref] [PubMed]
- Kim S, Bae SS, Chu HJ, et al. Capsule Endoscopy with Retention of the Capsule in a Duodenal Diverticulum: A Case Report. Korean J Gastroenterol 2016;67:207-11. [Crossref] [PubMed]