Recent advancements in stent therapy in patients with malignant gastroduodenal outlet obstruction
Introduction
Gastric outlet obstruction (GOO) leads to refractory vomiting, nausea, and poor oral food intake caused by tumors growing around the duodenum in those with pancreatic, biliary tract, duodenal and gastric cancer. In addition to such abdominal cancers, extra-abdominal cancers with lymph node metastasis and peritoneal dissemination of advanced cancers can cause malignant GOO. Surgical bypass traditionally has been a main treatment for malignant GOO and has been useful and feasible, especially in patients with a good performance status (1). However, performing a surgical procedure in end-stage patients is sometimes difficult.
Recently, the endoscopic placement of self-expandable metallic stents (SEMSs) has been developed as a viable treatment for malignant GOO as an alternative to surgical bypass. Endoscopic placement of a SEMS for patients with malignant GOO is less invasive, can shorten the duration of hospitalization, and enable patients to resume oral intake earlier in comparison with a surgical procedure (2-4).
Many reports have referred to the feasibility, effectiveness, and safety of the deployment of SEMSs for malignant GOO. Recently, in addition to these reports, several reports have described differences in clinical results among the kinds of SEMSs and predictive factors for successful resumption of oral intake after deployment of a SEMS. We herein review the current literature concerning endoscopic treatment using SEMSs for patients with malignant GOO.
Clinical results
Table 1 shows clinical results of prospective and multicenter studies of endoscopic treatment using SEMS for patients with malignant GOO. These reports show a technical success rate for the endoscopic deployment of a SEMS in patients with malignant GOO ranging from 95–100% (Table 1) (5-18). Reasons for unsuccessful deployment of a SEMS included unsuccessful passage of the guidewire or stent delivery system because of severity of the stricture (8,12), perforation during the procedure (9,16), insufficient deployment (11), and functional problems with SEMSs (6). Though definitions of clinical success differ slightly among studies, the rates of clinical success, which are indicated by an improvement in the GOO ranged from 77–94% (5-18). Median survival after deployment of a SEMS was reported to be approximately 3 months or less in most reports. Though Endo et al. reported a longer survival period, all of their study patients had gastric cancer, which might account for this result (15).

Full table
Adverse events
The reported rate for adverse events including stent dysfunction after deployment of SEMS for patients with malignant GOO is 15–48% (Table 1) (5-18). The rates of stent dysfunction and other adverse events are 5-40% and 0–23%, respectively. Though the rate of stent dysfunction is less than 30% in most reports, the rate reported Nassif et al. was 40% (5). In this report, “primary stent dysfunction” defined as unsuccessful SEMS dilation occurred in 8 patients (13%). However, unsuccessful dilation of a SEMS is no longer a frequent adverse event because of recent progress in the function of SEMSs.
Bleeding and perforation are severe adverse events associated with mortality; however, few reports exist regarding fatal bleeding after deployment of a SEMS. Matsumoto et al. reported a fatal case due to massive gastrointestinal tract bleeding on day 43 after deployment of SEMS (19). They concluded that the disruption of the artery occurred in the necrotic portion of the tumor caused by SEMS deployment and bacterial infection. We need to take precautions against massive bleeding caused by mechanical pressure as a late complication after SEMS deployment in cases of a tumor involving an artery. Ge et al. described delayed migration of a WallFlex enteral stent with subsequent visceral perforation four months after SEMS deployment, which was connected with shrinking of the tumor by chemotherapy (20). Several reports did not find evidence of perforation as an adverse event (8,10-12,14,18), and the risk of perforation is thought to be low. However, when it occurs perforation is directly associated with mortality, therefore, special attention should be paid to the possibility of perforation with a SEMS. As to another rare adverse event, Javaid et al. reported a case of fracture of a covered SEMS (21). The position of placement of a SEMS is sometimes associated with adverse events. Liu et al. reported the rate of acute pancreatitis in patients undergoing SEMS placement across the duodenal papilla at the rate of 11% (9/35) (22). Multivariate analysis revealed that the presence of a stent bridging the duodenal papilla [odds ratio (OR) =18.48; 95% CI, 2.298–148.48; P=0.006] was an independent predictor of acute pancreatitis.
Covered metallic stents (CMS) vs. uncovered metallic stents (UCMS)
Table 2 shows the results of comparisons between CMS and UCMS in a prospective study and four randomized controlled trials (23-27). The technical and clinical success rates were similar between CMS and UCMS in all of these reports. As an adverse event, the migration rate was significantly lower in the UCMS group in three of these five reports (23-25). However, Maetani et al. reported that there was no significant difference in the migration rate between CMS and UCMS because the 15-mm uncovered portion at both ends of the UCMS prevented stent migration (26). Lee et al. also reported no significant difference in the migration rate between CMS and UCMS because of the anti-migration properties of CMS (27). On the other hand, the rate of tumor ingrowth or overgrowth in CMS was significantly lower than for UCMS in all reports shown in Table 2, which is an advantage of CMS. However, the rates of re-intervention and stent patency between CMS and UCMS were similar in these reports except for that of Lee et al. (27). Lee et al. reported that the CMS group had a significantly longer cumulative duration of stent patency compared with the UCMS group (27), though all patients undergoing deployment of SEMS had gastric cancer. Minata et al. reviewed several studies about comparison between CMS and UCMS. There was a higher migration rate in CMS compared to UCMS in the palliation of malignant GOO. Nevertheless, covered SEMS had lower obstruction rates. There was no significant difference in technical success, clinical success, complications, bleeding, perforation, stent fracture and need for reintervention (28).
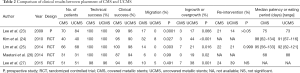
Full table
Metallic stents with different axial forces
The patency of a SEMS deployed for patients with biliary stricture is related to its axial force, which is connected with kinking of the bile duct (29). Similarly, the use of SEMSs with a low axial force for patients with malignant GOO is thought to be likely to decrease the risk of stent dysfunction caused by kinking because of the angulation of the duodenum. Okuwaki et al. compared two types UCMS with different axial forces: a Niti-S pyloric/duodenal stent (Taewoong Medical, Gimpo, Korea) and a WallFlex duodenal stent (Boston Scientific, Marlborough, MA, USA) (30). The Niti-S pyloric/duodenal stent is thought to have lower axial force. The median time to recurrent duodenal obstruction was significantly longer in the Niti-S group than in the WallFlex group, and the incidence of stent dysfunction was lower in the Niti-S group. Kato et al. compared the same two types of UCMS as did Okuwaki et al. (17). Though there was no significant difference in patency and the rate of stent dysfunction between the two groups, the clinical success rate in the Niti-S group was significantly higher than in the WallFlex group. However, the survival period between the two groups was similar in both studies. Because of the small number of patients in the currently available studies or their retrospective design, a larger prospective clinical trial is needed to confirm the superiority of using SEMSs with lower axial force for duodenal obstruction.
Predictive factors for clinical success
Despite of high successful rate of technical success of SEMS placement for the patients with malignant GOO, it is a problem to be resolved that all patients with technical success cannot achieve clinical success. Several reports concluded that performance status is a dependent predictive factor associated with clinical success (31-34). Yamao et al. reported that three or more stenosis sites (OR =6.11; P<0.01) predicted clinical success in addition to the performance status. Several reports reported that the presence of ascites or peritoneal dissemination was thought to be a predictive factor for the unsuccessful resumption of oral intake after the deployment of SEMS (31,32,34,35). However, there are differences among these reports. Sasaki et al. reported that not carcinomatosis but ascites (OR =3.28; 95% CI, 1.23–9.05; P=0.02) was a predictor associated with resumption of oral intake. On the other hands, Hori et al. and Sato et al. concluded that not ascites but carcinomatosis was a dependent predictive factor associated with clinical success. Mendelsohn et al. analyzed the results of placement of SEMS for malignant GOO in patients with or without carcinomatosis and concluded that there were no statistically significant differences between the two groups with regard to clinical outcomes or reintervention rates (P=0.95, 0.34, respectively). In addition, there was no statistically significant difference in the rate of clinical success between carcinomatosis patients with no/small ascites and those with moderate/severe ascites (P=0.7) (36).
Results of reinterventions
Few reports have analyzed the results of reinterventions for stent dysfunction after placement of SEMSs for malignant GOO. Stent dysfunction occurred in the range of 5–30% in patients with SEMS placement (Table 1). Kim et al. reported the results of stent-in-stent placements performed in 48 patients with stent dysfunction (37). The technical success rate and the clinical success rate were 97.9% and 95.8%, respectively. The median patency was 27.4 weeks (IQR, 21.6–51.0 weeks). No adverse events in addition to stent dysfunction occurred. Sato et al. reported technical and clinical success rates of 100% and 85.7%, respectively, for stent-in-stent placements performed in 14 patients (32). Median stent patency was 172 days (range, 4–205 days). Although no severe complications such as massive bleeding and perforation were shown in those reports, Sasaki et al. reported a high rate of severe adverse events (38). The technical success rate and the clinical success rate were 100% and 86.2%, respectively, in the 29 patients undergoing stent-in-stent placement of a secondary stent. The median oral intake period was 3.0 months (95% CI, 2.1–4.1 months). As a severe adverse event, gastrointestinal perforation occurred at the rate of 13.8%, which was quite high. The authors hypothesized that some SEMS properties (e.g., axial force) might be enhanced when secondary gastroduodenal SEMSs are placed by the stent-in-stent technique. They noted that one way to prevent gastrointestinal perforation was to choose a lower axial-force SEMS for a gastroduodenal SEMS inserted at the bending site (supraduodenal angle or intraduodenal angle) as a secondary stent.
Conclusions
The success rate for the placement of a SEMS for malignant GOO was sufficient. The rate of adverse events is admissible, however, severe adverse events sometimes occur. UCMS are preferable to CMS in avoiding stent migration, while CMS is preferable to UCMS in avoiding tumor ingrowth or overgrowth. Selection of a SEMS with a lower axial force may be important to achieve long patency and to avoid gastroduodenal perforation at the bending site of the duodenum. Clinical success rates were usually lower than technical success rates and a predictive factor for failure of clinical success is lower performance status. The role of other factors such as carcinomatosa and ascites remains controversial. Both technical and clinical success rates for placement of a secondary SEMS after dysfunction of a previously placed stent is similar to those for placement of the first SEMS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- No JH, Kim SW, Lim CH, et al. Long-term outcome of palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer in patients with good performance status: endoscopic stenting versus surgery. Gastrointest Endosc 2013;78:55-62. [Crossref] [PubMed]
- Espinel J, Sanz O, Vivas S, et al. Malignant gastrointestinal obstruction: endoscopic stenting versus surgical palliation. Surg Endosc 2006;20:1083-7. [Crossref] [PubMed]
- Khashab M, Alawad AS, Shin EJ, et al. Enteral stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Surg Endosc 2013;27:2068-75. [Crossref] [PubMed]
- Park JH, Song HY, Yun SC, et al. Gastroduodenal stent placement versus surgical gastrojejunostomy for the palliation of gastric outlet obstructions in patients with unresectable gastric cancer: a propensity score-matched analysis. Eur Radiol 2016;26:2436-45. [Crossref] [PubMed]
- Nassif T, Prat F, Meduri B, et al. Endoscopic palliation of malignant gastric outlet obstruction using self-expandable metallic stents: results of a multicenter study. Endoscopy 2003;35:483-9. [Crossref] [PubMed]
- Telford JJ, Carr-Locke DL, Baron TH, et al. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest Endosc 2004;60:916-20. [Crossref] [PubMed]
- Graber I, Dumas R, Filoche B, et al. The efficacy and safety of duodenal stenting: a prospective multicenter study. Endoscopy 2007;39:784-7. [Crossref] [PubMed]
- Kim JH, Song HY, Shin JH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc 2007;66:256-64. [Crossref] [PubMed]
- Maetani I, Isayama H, Mizumoto Y. Palliation in patients with malignant gastric outlet obstruction with a newly designed enteral stent: a multicenter study. Gastrointest Endosc 2007;66:355-60. [Crossref] [PubMed]
- Piesman M, Kozarek RA, Brandabur JJ, et al. Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol 2009;104:2404-11. [Crossref] [PubMed]
- van Hooft JE, Uitdehaag MJ, Bruno MJ, et al. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc 2009;69:1059-66. [Crossref] [PubMed]
- van Hooft JE, van Montfoort ML, Jeurnink SM, et al. Safety and efficacy of a new non-foreshortening nitinol stent in malignant gastric outlet obstruction (DUONITI study): a prospective, multicenter study. Endoscopy 2011;43:671-5. [Crossref] [PubMed]
- Costamagna G, Tringali A, Spicak J, et al. Treatment of malignant gastroduodenal obstruction with a nitinol self-expanding metal stent: an international prospective multicentre registry. Dig Liver Dis 2012;44:37-43. [Crossref] [PubMed]
- Sasaki T, Isayama H, Maetani I, et al. Japanese multicenter estimation of WallFlex duodenal stent for unresectable malignant gastric outlet obstruction. Dig Endosc 2013;25:1-6. [Crossref] [PubMed]
- Endo S, Takiguchi S, Miyazaki Y, et al. Efficacy of endoscopic gastroduodenal stenting for gastric outlet obstruction due to unresectable advanced gastric cancer: a prospective multicenter study. J Surg Oncol 2014;109:208-12. [Crossref] [PubMed]
- Miyabe K, Hayashi K, Nakazawa T, et al. Safety and benefits of self-expandable metallic stents with chemotherapy for malignant gastric outlet obstruction. Dig Endosc 2015;27:572-81. [Crossref] [PubMed]
- Kato H, Kawamoto H, Matsumoto K, et al. Outcome of self-expandable metallic stent deployment in patients with malignant gastroduodenal outlet obstruction and Niti-S and WallFlex comparison: a multicenter retrospective clinical study. J Dig Dis 2016;17:518-525. [Crossref] [PubMed]
- Sasaki R, Sakai Y, Tsuyuguchi T, et al. Endoscopic management of unresectable malignant gastroduodenal obstruction with a nitinol uncovered metal stent: A prospective Japanese multicenter study. World J Gastroenterol 2016;22:3837-44. [Crossref] [PubMed]
- Matsumoto K, Hayashi A, Yashima K, et al. Late complications of self-expandable metallic stent placement for malignant gastric outlet obstruction. Intern Med 2014;53:2773-5. [Crossref] [PubMed]
- Ge PS, Watson RR, Chen DC, et al. Delayed Migration of a WallFlex Enteral Stent Resulting in Jejunal Perforation. Case Rep Gastrointest Med 2013;2013:652597.
- Javaid MR, Yusuf AM. An instant rare complication: a fractured metallic pyloric stent. BMJ Case Rep 2013;2013.
- Shi-Yi L, Ai-Wu M, Yi-Ping J, et al. Placement of duodenal stents across the duodenal papilla may predispose to acute pancreatitis: a retrospective analysis. Diagn Interv Radiol 2012;18:360-4. [PubMed]
- Lee KM, Choi SJ, Shin SJ, et al. Palliative treatment of malignant gastroduodenal obstruction with metallic stent: prospective comparison of covered and uncovered stents. Scand J Gastroenterol 2009;44:846-52. [Crossref] [PubMed]
- Kim CG, Choi IJ, Lee JY, et al. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc 2010;72:25-32. [Crossref] [PubMed]
- Lim SG, Kim JH, Lee KM, et al. Conformable covered versus uncovered self-expandable metallic stents for palliation of malignant gastroduodenal obstruction: a randomized prospective study. Dig Liver Dis 2014;46:603-8. [Crossref] [PubMed]
- Maetani I, Mizumoto Y, Shigoka H, et al. Placement of a triple-layered covered versus uncovered metallic stent for palliation of malignant gastric outlet obstruction: a multicenter randomized trial. Dig Endosc 2014;26:192-9. [Crossref] [PubMed]
- Lee H, Min BH, Lee JH, et al. Covered metallic stents with an anti-migration design vs. uncovered stents for the palliation of malignant gastric outlet obstruction: a multicenter, randomized trial. Am J Gastroenterol 2015;110:1440-9. [Crossref] [PubMed]
- Minata MK, Bernardo WM, Rocha RS, et al. Stents and surgical interventions in the palliation of gastric outlet obstruction: a systematic review. Endosc Int Open 2016;4:E1158-E1170. [Crossref] [PubMed]
- Isayama H, Nakai Y, Toyokawa Y, et al. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointest Endosc 2009;70:37-44. [Crossref] [PubMed]
- Okuwaki K, Kida M, Yamauchi H, et al. Randomized controlled exploratory study comparing the usefulness of two types of metallic stents with different axial forces for the management of duodenal obstruction caused by pancreatobiliary cancer. J Hepatobiliary Pancreat Sci 2016;23:289-97. [Crossref] [PubMed]
- Sasaki T, Isayama H, Nakai Y, et al. Predictive factors of solid food intake in patients with malignant gastric outlet obstruction receiving self-expandable metallic stents for palliation. Dig Endosc 2012;24:226-30. [Crossref] [PubMed]
- Sato T, Hara K, Mizuno N, et al. Gastroduodenal stenting with Niti-S stent: long-term benefits and additional stent intervention. Dig Endosc 2015;27:121-9. [Crossref] [PubMed]
- Yamao K, Kitano M, Kayahara T, et al. Factors predicting through-the-scope gastroduodenal stenting outcomes in patients with gastric outlet obstruction: a large multicenter retrospective study in West Japan. Gastrointest Endosc 2016;84:757-763.e6. [Crossref] [PubMed]
- Hori Y, Naitoh I, Ban T, et al. Stent under-expansion on the procedure day, a predictive factor for poor oral intake after metallic stenting for gastric outlet obstruction. J Gastroenterol Hepatol 2015;30:1246-51. [Crossref] [PubMed]
- Shin YS, Choi CW, Kang DH, et al. Factors associated with clinical failure of self-expandable metal stent for malignant gastroduodenal obstruction. Scand J Gastroenterol 2016;51:103-10. [Crossref] [PubMed]
- Mendelsohn RB, Gerdes H, Markowitz AJ, et al. Carcinomatosis is not a contraindication to enteral stenting in selected patients with malignant gastric outlet obstruction. Gastrointest Endosc 2011;73:1135-40. [Crossref] [PubMed]
- Kim CG, Choi IJ, Lee JY, et al. Outcomes of second self-expandable metallic stent insertion for malignant gastric outlet obstruction. Surg Endosc 2014;28:281-8. [Crossref] [PubMed]
- Sasaki T, Isayama H, Nakai Y, et al. Clinical outcomes of secondary gastroduodenal self-expandable metallic stent placement by stent-in-stent technique for malignant gastric outlet obstruction. Dig Endosc 2015;27:37-43. [Crossref] [PubMed]


