Optimizing antiviral agents for hepatitis B management in malignant lymphomas
Prevalence of chronic hepatitis B (CHB) by region, age group and special groups (lymphoma)
The hepatitis B virus (HBV) is a common human infection which belongs to the hepadnaviridae family. The chronic form of HBV infection is more prevalent than the acute type, and global estimate of chronic cases is 300 million or more (1-3). Despite the likelihood that 95% of adults will recover from an acute HBV infection, impaired viral clearance facilitates complications such as chronic hepatic inflammation, hepatocellular carcinoma and lymphomas (4-7). On a global scale, the mortality from HBV complications is estimated at about 780,000–1 million yearly and adds to existing burdens to health systems (2,8). Global estimates of CHB prevalence indicate high prevalence of CHB in the African and Western pacific region and countries with the highest number of HBsAg-positive individuals include China (74 million), India (17 million) and Nigeria (15 million) (9). Significant decrease in CHB prevalence has been recorded over the last 5 decades especially in countries located in South East Asia, Western Pacific, and Eastern Mediterranean regions. However, prevalence rates are increasing in parts of Europe partly as a result of migrant influx from highly endemic countries (10,11). In addition, vaccination efforts in parts of Europe are focused on high risk groups rather than the WHO recommendation of universal vaccination of newborns (12). HBsAg prevalence has been consistently low in countries such as Japan, Western Europe, Australia and most countries in the Americas as a result of early vaccination (9).
There are certain groups of patients that will benefit most from screening and monitoring assays of HBsAg, anti-HBc and HBV DNA. This group includes children, pregnant women, patients with acute and fulminant hepatitis B, decompensated cirrhosis, co-existing chronic renal disease, transplant subjects, and cancer patients on chemo-immunotherapy (13-15). These patients are more vulnerable to fatal hepatitis or HBV reactivation and are usually excluded from clinical trials (13,14). They constitute a majority of those with CHB and require concerted research on optimizing antiviral therapy among this group of patients (13,14). Individuals with negative HBsAg assay are not completely free of the risk of reactivation of hepatitis since viral DNA and anti-HBc may persist as an occult hepatitis B infection (OBI) (16-18). Presence of OBI undermines chronic HBV screening methods based on a positive HBsAg alone. Undiagnosed OBI also limits the prescription of antiviral agents that would have benefited this special group of patients in a timely manner. Malignant lymphoma patients are the group of focus in this review because studies have demonstrated an association between HBV and lymphoma etiology, likewise, lymphoma treatment outcomes (7,15,19). In addition, studies have demonstrated hepatitis B viral markers detectable in both Hodgkin’s and non-Hodgkin’s lymphomas with a propensity for non-Hodgkin’s lymphoma, and the diffuse B-cell lymphoma subtype (5,15,20-26).
This review is also critical because lymphoma therapy has evolved rapidly and resulted in improved survival over the last decade (26-28). However, this success in lymphoma therapy is undermined by the influence of chronic HBV on survival outcomes among these patients who tend to be immunosuppressed (29-31). Therefore, lymphoma patients with hepatitis B are a special group of patients that require optimizing HBV antivirals to improve treatment outcomes. The goal of this review is to evaluate the literature for optimizing antiviral therapy for lymphoma patients with HBV infection or at risk of HBV reactivation.
Review criteria
Relevant articles for this review were identified by searching PubMed, Embase, Ovid Medline, and Scopus using the following terms, alone and in combination: “chronic hepatitis B”, “occult hepatitis B”, ”special groups”, “malignant lymphoma”, “Non-Hodgkin’s lymphoma”, “Hodgkin’s lymphoma”, “immunocompromised host”, “immunosuppressive agents”, “antiviral”, “HBV reactivation”. Full text articles of all selected studies were retrieved, and if a paper was selected for inclusion, the bibliographic references were scrutinized to identify additional relevant studies. The period of the search was restricted to a 15-year period to limit the search to optimizing current antiviral agents for HBV infection in malignant lymphomas [2001–2016].
Mechanism of HBV infection & chronicity
The liver has numerous functions that include energy metabolism and immunity (32,33). A dysfunction in any of these processes results in metabolic impairment and more importantly, chronic hepatocyte inflammation, lymphomas and hepatocellular malignancies (34). Studies across countries (Asia, Europe, Australia, and United States) have demonstrated a possible causal association between chronic HBV infection and risk of lymphoma with an estimated odds ratio ranging from 1.5–3.6 (5,15,35,36). Once HBV infection occurs with or without evidence of seroconversion, it persists in the body, exposing certain individuals to risk of chronic infection, acute flare, reactivation or fulminant hepatitis (37). The HBV is transmitted through contact with infected blood, semen, and other body fluids; primarily through perinatal transmission from an infected mother, sexual contact with an infected person, sharing of contaminated needles, syringes, or other injection drug equipment, needle sticks or other sharp instrument injuries (38).
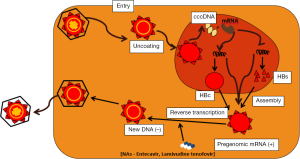
Once the virus reaches the body fluids of a new host, the virus invades the susceptible host hepatocytes where it fuses with the cellular membrane and releases its complementary DNA (cccDNA) for replication in the hepatocyte nucleus. Transcription occurs in a reverse manner with negative strand synthesis preceding positive strand synthesis. Subsequently, the virion receives its coat protein (HBsAg) in the cytoplasm before budding and secretion into the host blood (39,40) (Figure 1).
Key step that have been targeted in the viral replication process is the reverse transcription phase where viral maturation and replication is inhibited. Novel agents are undergoing clinical trials to target cccDNA which tends to persist in host, largely accounting for chronicity (39,40). Evolution in the understanding of the viral replication process continues to drive development of current and novel antivirals in different phases of clinical trials.
Diagnosis of CHB and parameters
It is important to make a clear distinction among viral markers used to aid diagnosis of CHB infection and its variants such as OBI. Markers of acute or CHB in a susceptible host include HBsAg and anti-HBs, HBeAg and anti-HBe, and anti-HBc IgM and IgG, HBV DNA (41); the presence of any of this signifies important physiological or disease states. Acute infection is indicated by presence of antigens, HBsAg and/or HBeAg, followed by the antibody, anti-HBc IgM. Early convalescence is indicated by the antibody, anti-HBc IgM which is cleared out in most patients. Late convalescence is indicated by elevated anti-HBc IgG or anti-HBs. Convalescence may then transit into chronicity which is confirmed by persistently elevated titers (≥6 months) of anti-HBc IgG, HBsAg, and or anti-HBe. Currently available serological assays now utilize HBV DNA levels to diagnose occult HBV, HBV reactivation in lymphoma chemo-immunotherapy (or cancer immunotherapy), HBV resistance during antiviral therapy, or HBeAg seroconversion during chronic HBV (accompanied by a sudden increase in serum ALT and HBV DNA) (Table 1).
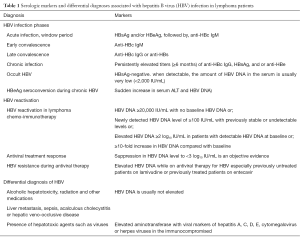
Full table
Occult HBV infection
The chronic form of HBV infection is facilitated by the persistence of covalently closed circular DNA (cccDNA) (42). The term occult HBV infection represents the absence of detectable serum HBsAg (resolved HBV infection) following immune-mediated control of viral replication but persistent viral genomes in the liver (43). The occult form of HBV emerges following a progressive disappearance of the hepatitis surface antigen over the years post-infection (43). The European Association of Liver Disease [2008] endorsed the definition of occult HBV as the presence of HBV DNA in the liver of individuals testing HBsAg-negative using currently available assays (16). When detectable, the amount of HBV DNA in the serum is usually very low (<2,000 IU/mL) (44,45). Occult HBV is clinically relevant considering silent transmission routes such as during blood transfusion, hemodialysis, organ transplant or potential reactivation during immunosuppressive therapy (43,46-48). Other factors that affect the natural history of CHB include host factors (gender, age at infection, family history), viral factors (viral mutation, genotype and HBV DNA level), and environmental factors (co-infection with hepatotrophic viruses, parasites or toxins) (49). Currently, there is no standard screening method for occult HBV infection except testing HBV PCR/DNA in high risk patients, neither does any society recommend HBV DNA screening for low risk patients at the current time.
HBV in lymphoma patients
Lymphoma patients are at risk of HBV reactivation due to a number of factors but individuals with inactive or occult forms of HBV are at greater risk of reactivation. One factor is lymphomagenesis which provides a milieu of immunosuppression which favors HBV replication. In addition (50,51), when immunosuppressive agents are initiated in lymphoma, induced immunosuppression triggers viral replication that manifests as HBV reactivation (38,52-54). It is important to note that spontaneous reactivation can occur in individuals without lymphoma, especially individuals with positive HBeAg, likewise co-infection with HIV, bacterial infections or stressors (emotional and physical) (55). A common underlying mechanism is the role of altered patient immune response (55). Heredity or acquired genetic mutations also play a critical role in HBV marker persistence, response to antiviral therapy and risk of NHL (20,56-60). Case-control studies among European patients with NHL have demonstrated that individuals with dysfunction in WBC telomere length have a higher risk of B-cell lymphoma (61-63). Among patients with lymphoma and concurrent HBV reactivation, a key environmental factor includes specific drug classes. These drugs include chemotherapeutic agents, with or without combination of anti-CD20 agents, anti-CD52 agents, immunosuppressive (methotrexate or azathioprine) or glucocorticoids (64-68). Additional biologic agents such as TNF inhibitors and tyrosine kinase inhibitors have been linked to HBV reactivation but are not in use for lymphoma therapy currently.
Risk stratification of HBV reactivation
The general classification of HBV reactivation is based on HBV biomarkers, type of immunosuppressive therapy and/or use of glucocorticoid combined therapy (64-70). Among patients with a positive HBsAg or anti-HBc biomarkers, a very high risk of HBV reactivation (>20%) is associated with anti-CD20 monoclonal antibody agents (rituximab, obinutuzumab, ofatumumab) (37,50,64-68,71-73). The risk may be higher with the presence of HBeAg and/or elevated baseline HBV DNA (74-76). When HBsAg-positive patients are to receive high-dose glucocorticoids (>20 mg/day for ≥4 weeks), they are considered at high risk for reactivation (11–20%). Moderate risk (1–10%) is present where a patient is HBsAg positive and is going to be placed on cytotoxic therapy such as cyclophosphamide, doxorubicin, vincristine, excluding glucocorticoids. Low risk patients (<1%) includes two types of patients: HBsAg-positive patient who is going to receive either methotrexate or azathioprine; HBsAg-negative and anti-HBc-positive patients going to receive high-dose glucocorticoids or anti-CD52 agent, alemtuzumab (77). The risk for reactivation is lowest for HBsAg negative and anti-HBc-positive patients receiving non-glucocorticoid-based chemotherapy, azathioprine or methotrexate (77).
Clinical features of reactivation
Most cases of HBV reactivation do not have symptoms and the only indicator is an elevated HBV DNA level (76,78,79). Fewer patients with HBV reactivation may present with increased aminotransferase levels, with or without mild features such as nausea and vomiting. Grave clinical features include icterus, decompensated hepatic function, or mortality, especially with existing cirrhosis (53,56,80).
Serology of HBV reactivation and differential diagnoses in lymphoma patients
In order to make a diagnosis of HBV reactivation, there should be serologic evidence of HBV (71,81):
- HBV DNA ≥20,000 IU/mL with no baseline HBV DNA or;
- Newly detected HBV DNA level of ≥100 IU/mL with previously stable or undetectable levels or;
- Elevated HBV DNA ≥2 log10 IU/mL in patients with detectable HBV DNA at baseline or;
- ≥10-fold increase in HBV DNA compared with baseline.
When making a diagnosis of HBV reactivation it is crucial to differentiate reactivation from the following conditions: acute HBV (elevated IgM anti-HBc titer), HBeAg seroconversion during chronic HBV (accompanied by a sudden increase in serum ALT and HBV DNA), HBV resistance (elevated HBV DNA while on antiviral therapy for HBV especially previously untreated patients on lamivudine or previously treated patients on entecavir), presence of hepatotoxic agents such as viruses (elevated aminotransferase with viral markers of hepatitis A, C, D, E, cytomegalovirus or herpes viruses in the immunocompromised). Clinical manifestation of hepatitis may be present in other conditions such as alcoholic hepatotoxicity, radiation and other medications but HBV DNA is usually not elevated. Lastly, other liver diseases need to be ruled out in lymphoma patients and these include liver metastasis, sepsis, acalculous cholecystitis or hepatic veno-occlusive disease (77).
Antivirals in CHB and lymphoma
There are currently two treatment modalities for chronically infected HBV patients that include nucleot(s)ide analogs and interferon alpha. Interferons are not currently prescribed for lymphoma patients with HBV reactivation because of the associated intolerance, adverse reactions and selective effectiveness (40). The majority of infected patients require lifelong therapy, yet viral rebound commonly occurs following termination of therapy. Nevertheless, antivirals are recommended for all lymphoma patients with HBV reactivation and those with acute HBV or infection from other hepatotropic viruses. The focus of this review is solely on optimizing antiviral therapy for HBV among patients with lymphoma; therefore, other differential diagnoses enumerated above will require extensive workup to determine a suitable therapy.
Drug targets in viral infection cycle/phase
The reverse transcription phase of the viral life cycle in susceptible hosts is the most successfully targeted phase. The reverse transcriptase enzyme synthesizes double stranded DNA (dsDNA) from host RNA, and the synthesized dsDNA integrates into the host genome. The nucleos(t)ide analogs are taken up by hepatocytes, converted by viral and cellular enzymes to a form which competitively block nucleotide binding to reverse transcriptase, and polymerase enzymes (DNA/RNA), thereby terminating normal DNA chain formation. Lamivudine requires phosphorylation while tenofovir requires phosphorylation to be activated. The result of the blocking action is prevention of virion assembly and maturation. Lamivudine and Tenofovir are the main antivirals in use in HBV infection or reactivation. Non-nucleos(t)ide polymerase inhibitors bind the reverse transcriptase site at a different site from nucleoside reverse transcriptase inhibitor (NRTI) and also prevent viral maturation and release.
Antiviral therapy for lymphoma patients with HBV also target the reverse transcription phase of the viral life cycle thereby limiting viral maturation and replication (Figure 1). More importantly, currently available antiviral therapies do not target defective responses of the immune system or the persistence of covalently closed circular DNA (cccDNA) in the infected hepatocytes. Thus, developing different approaches to achieve sustained cure or elimination of HBV is urgently needed.
Guidelines & summary of evidence for effective antiviral agents in lymphoma patients
Several clinical practice guidelines recommend nucleos(t)ide analogues entecavir, tenofovir and lamivudine among others. These agents are best initiated along with or prior to immunosuppressive therapy. The timing and choice of therapy depends on the level of risk, planned duration of chemotherapy, HBV-DNA level, and prior antiviral therapy (82-87). High risk patients ought to receive antiviral therapy preferably before or simultaneously with potent immunosuppressive agents. Entecavir and tenofovir are first line agents according to most studies and guidelines (55,82,85,86), however, lamivudine may be used if chemotherapy will last less than 6 months, HBV-DNA (<2,000 IU/mL), the patient is treatment-naïve and first line agents are impossible to obtain (55,82,84,88). For patients with prior lamivudine therapy, tenofovir may be more suitable than entecavir after determining HBV resistance (37,89). Patients determined to be at lower risk would suffice on close monitoring of HBV DNA levels instead of preventive antiviral therapy.
Antiviral therapy should continue for at least 6 months post-chemotherapeutic agents and extended to a minimum of 12 months when the anti-CD20 agent, rituximab is administered (82,85,86). Suppression in HBV DNA level to <3 log10 IU/mL is an objective evidence of antiviral treatment response and may be an appropriate time to initiate immunosuppressive agents among patients with significantly elevated HBV DNA (37,88).
First-line therapy for chronic HBV in lymphoma patients is predominantly entecavir, tenofovir and lamivudine, yet, the optimal workup for individual patients differ. Some guidelines now support inclusion of hepatitis specialists in managing these patients (84,89). Additional methods recommended for optimizing antiviral therapy include laboratory modalities such as HBV genotyping, timed measurements of HBsAg and HBV DNA levels to measure and predict antiviral treatment response (55,82,85,87,88,90).
Conclusions
Lymphoma patients are special groups of individuals at risk of adverse outcomes from co-existing HBV infection. All lymphoma patients do not carry the same level of HBV reactivation risk from exposure to immunosuppressive agents. Lymphoma patients on rituximab therapy and are positive for HBsAg have the highest risk of HBV reactivation. First line antiviral agents recommended include entecavir, lamivudine and tenofovir. Optimizing antiviral agents for these patients require consideration of geographic prevalence of HBV, cost of antiviral therapy or testing, screening modality, hepatitis experts, type of immunosuppressive therapy and planned duration of therapy. A minimum period of 6 months is recommended for antiviral therapy but the time to stop therapy is unclear. Lastly, efforts to increase CHB screening and therapy need to be sustained to improve treatment outcomes for lymphoma patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization. Hepatitis B. WHO Fact Sheet No. 204. 2016. Available online: http://www.who.int/mediacentre/factsheets/fs204/en/
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [Crossref] [PubMed]
- Goldstein ST, Zhou F, Hadler SC, et al. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005;34:1329-39. [Crossref] [PubMed]
- Czaja MJ, Ding WX, Donohue TM Jr, et al. Functions of autophagy in normal and diseased liver. Autophagy 2013;9:1131-58. [Crossref] [PubMed]
- Nath A, Agarwal R, Malhotra P, et al. Prevalence of hepatitis B virus infection in non-Hodgkin lymphoma: a systematic review and meta-analysis. Intern Med J 2010;40:633-41. [Crossref] [PubMed]
- Bumbea H, Vladareanu AM, Vintilescu A, et al. The lymphocyte immunophenotypical pattern in chronic lymphocytic leukemia associated with hepatitis viral infections. J Med Life 2011;4:256-63. [PubMed]
- Ozoya OO, Sokol L, Dalia S, Hepatitis B. Reactivation with Novel Agents in Non-Hodgkin’s Lymphoma and Prevention Strategies. J Clin Transl Hepatol 2016;4:143-50. [PubMed]
- Dienstag JL. Hepatitis B virus infection. N Engl J Med 2008;359:1486-500. [Crossref] [PubMed]
- Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546-55. [Crossref] [PubMed]
- Rossi C, Shrier I, Marshall L, et al. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta-analysis. PloS One 2012;7:e44611. [Crossref] [PubMed]
- Chu JJ, Wörmann T, Popp J, et al. Changing epidemiology of hepatitis B and migration--a comparison of six Northern and North-Western European countries. Eur J Public Health 2013;23:642-7. [Crossref] [PubMed]
- World Health Organization. Migration and health: key issues. 2016. Available online: http://www.euro.who.int/en/health-topics/health-determinants/migration-and-health/migrant-health-in-the-european-region/migration-and-health-key-issues#292117
- Rapti IN, Hadziyannis SJ. Treatment of special populations with chronic hepatitis B infection. Expert Rev Gastroenterol Hepatol 2011;5:323-39. [Crossref] [PubMed]
- Cholongitas E, Tziomalos K, Pipili C. Management of patients with hepatitis B in special populations. World J Gastroenterol 2015;21:1738-48. [Crossref] [PubMed]
- Dalia S, Chavez J, Castillo JJ, et al. Hepatitis B infection increases the risk of non-Hodgkin lymphoma: a meta-analysis of observational studies. Leuk Res 2013;37:1107-15. [Crossref] [PubMed]
- Said ZN. An overview of occult hepatitis B virus infection. World J Gastroenterol 2011;17:1927-38. [Crossref] [PubMed]
- Cheung WI. Prospective evaluation of seropositive occult hepatitis B viral infection in lymphoma patients receiving chemotherapy. Hong Kong Med J 2011;17:376-80. [PubMed]
- Elbedewy TA, Elashtokhy HE, Rabee ES, et al. Prevalence and chemotherapy-induced reactivation of occult hepatitis B virus among hepatitis B surface antigen negative patients with diffuse large B-cell lymphoma: significance of hepatitis B core antibodies screening. J Egypt Natl Canc Inst 2015;27:11-8. [Crossref] [PubMed]
- Dong HJ, Ni LN, Sheng GF, et al. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol 2013;57:209-14. [Crossref] [PubMed]
- Makvandi K, Ranjbari N, Makvandi M, et al. Study of the Association of Mutant HBsAg Gene and Hodgkin and Non-Hodgkin Lymphoma. Jundishapur J Microbiol 2015;8:e25726. [Crossref] [PubMed]
- Qi Z, Wang H, Gao G. Association of risk of non-Hodgkin's lymphoma with hepatitis B virus infection: a meta-analysis. Int J Clin Exp Med 2015;8:22167-74. [PubMed]
- Kleinstern G, Seir RA, Perlman R, et al. Associations between B-cell non-Hodgkin lymphoma and exposure, persistence and immune response to Hepatitis B. Haematologica 2016;101:e303-5. [Crossref] [PubMed]
- Abe SK, Inoue M, Sawada N, et al. Hepatitis B and C virus infection and risk of lymphoid malignancies: A population-based cohort study (JPHC Study). Cancer Epidemiol 2015;39:562-6. [Crossref] [PubMed]
- Kim MG, Park SY, Kim EJ, et al. Hepatitis B virus reactivation in a primary central nervous system lymphoma patient following intrathecal rituximab treatment. Acta Haematol 2011;125:121-4. [Crossref] [PubMed]
- Tajima K, Takahashi N, Ishizawa K, et al. High prevalence of diffuse large B-cell lymphoma in occult hepatitis B virus-infected patients in the Tohoku district in Eastern Japan. J Med Virol 2016;88:2206-2210. [Crossref] [PubMed]
- Cheson BD. CLL and NHL: the end of chemotherapy? Blood 2014;123:3368-70. [Crossref] [PubMed]
- Grover NS, Park SI. Novel Targeted Agents in Hodgkin and Non-Hodgkin Lymphoma Therapy. Pharmaceuticals (Basel) 2015;8:607-36. [Crossref] [PubMed]
- Siddiqi T, Rosen ST. Novel biologic agents for non-Hodgkin lymphoma and chronic lymphocytic leukemia-part 2: adoptive cellular immunotherapy, small-molecule inhibitors, and immunomodulation. Oncology (Williston Park) 2015;29:299-308. [PubMed]
- Morrison VA. Immunosuppression associated with novel chemotherapy agents and monoclonal antibodies. Clin Infect Dis 2014;59 Suppl 5:S360-4. [Crossref] [PubMed]
- Richey EA, Lyons EA, Nebeker JR, et al. Accelerated approval of cancer drugs: improved access to therapeutic breakthroughs or early release of unsafe and ineffective drugs? J Clin Oncol 2009;27:4398-405. [Crossref] [PubMed]
- Phipps C, Chen Y, Tan D. Lymphoproliferative Disease and Hepatitis B Reactivation: Challenges in the Era of Rapidly Evolving Targeted Therapy. Clin Lymphoma Myeloma Leuk 2016;16:5-11. [Crossref] [PubMed]
- Rui L. Energy metabolism in the liver. Compr Physiol 2014;4:177-97. [Crossref] [PubMed]
- Dixon LJ, Barnes M, Tang H, et al. Kupffer cells in the liver. Compr Physiol 2013;3:785-97. [PubMed]
- Dixon S, McDonald S, Roberts J. The impact of HIV and AIDS on Africa's economic development. BMJ 2002;324:232-4. [Crossref] [PubMed]
- Datta S, Chatterjee S, Policegoudra RS, et al. Hepatitis viruses and non-Hodgkin’s lymphoma: A review. World J Virol 2012;1:162-73. [Crossref] [PubMed]
- Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol 2010;11:827-34. [Crossref] [PubMed]
- Lok AS, McMahon BJ, Brown RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 2016;63:284-306. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). The ABCs of Hepatitis. 2015. Available online: https://www.cdc.gov/hepatitis/resources/professionals/pdfs/abctable.pdf
- Urban S, Schulze A, Dandri M, et al. The replication cycle of hepatitis B virus. J Hepatol 2010;52:282-4. [Crossref] [PubMed]
- Chen J, Wu M, Liu K, et al. New insights into hepatitis B virus biology and implications for novel antiviral strategies. Natl Sci Rev 2015;2:296-313. [Crossref]
- Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014;384:2053-63. [Crossref] [PubMed]
- Levrero M, Pollicino T, Petersen J, et al. Control of cccDNA function in hepatitis B virus infection. J Hepatol 2009;51:581-92. [Crossref] [PubMed]
- Raimondo G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection. J Hepatol 2007;46:160-70. [Crossref] [PubMed]
- Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008;49:652-7. [Crossref] [PubMed]
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661-2. [Crossref] [PubMed]
- Kwak MS, Kim YJ. Occult hepatitis B virus infection. World J Hepatol 2014;6:860-9. [Crossref] [PubMed]
- Liu Y, Li P, Li C, et al. Detection of hepatitis B virus DNA among accepted blood donors in Nanjing, China. Virol J 2010;7:193. [Crossref] [PubMed]
- Liu CJ, Chen DS, Chen PJ. Epidemiology of HBV infection in Asian blood donors: Emphasis on occult HBV infection and the role of NAT. J Clin Virol 2006;36:S33-S44. [Crossref] [PubMed]
- Croagh CM, Lubel JS. Natural history of chronic hepatitis B: phases in a complex relationship. World J Gastroenterol 2014;20:10395-404. [Crossref] [PubMed]
- Dalia S, Suleiman Y, Croy D, et al. Association of Lymphomagenesis and the Reactivation of Hepatitis B Virus in Non-Hodgkin Lymphoma. Cancer Control 2015;22:360-5. [PubMed]
- Shouval D, Shibolet O. Immunosuppression and HBV reactivation. Semin Liver Dis 2013;33:167-77. [Crossref] [PubMed]
- Hsu C, Tsou HH, Lin SJ, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology 2014;59:2092-100. [Crossref] [PubMed]
- Yeo W, Chan HL. Hepatitis B virus reactivation associated with anti-neoplastic therapy. J Gastroenterol Hepatol 2013;28:31-7. [Crossref] [PubMed]
- Chen KL, Chen J, Rao HL, et al. Hepatitis B virus reactivation and hepatitis in diffuse large B-cell lymphoma patients with resolved hepatitis B receiving rituximab-containing chemotherapy: risk factors and survival. Chin J Cancer 2015;34:225-34. [Crossref] [PubMed]
- Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98. [Crossref] [PubMed]
- Win LL, Powis J, Shah H, et al. Death from Liver Failure despite Lamivudine Prophylaxis during R-CHOP Chemotherapy due to Rapid Emergence M204 Mutations. Case Reports Hepatol 2013; 2013:454897.
- Sato T, Kato J, Kawanishi J, et al. Acute exacerbation of hepatitis due to reactivation of hepatitis B virus with mutations in the core region after chemotherapy for malignant lymphoma. J Gastroenterol 1997;32:668-71. [Crossref] [PubMed]
- Liu WC, Phiet PH, Chiang TY, et al. Five subgenotypes of hepatitis B virus genotype B with distinct geographic and virological characteristics. Virus Res 2007;129:212-23. [Crossref] [PubMed]
- Picardi M, Pane F, Quintarelli C, et al. Hepatitis B virus reactivation after fludarabine-based regimens for indolent non-Hodgkin's lymphomas: high prevalence of acquired viral genomic mutations. Haematologica 2003;88:1296-303. [PubMed]
- Cerhan JR, Berndt SI, Vijai J, et al. Genome-wide association study identifies multiple susceptibility loci for diffuse large B cell lymphoma. Nat Genet 2014;46:1233-8. [Crossref] [PubMed]
- Machiela MJ, Lan Q, Slager SL, et al. Genetically predicted longer telomere length is associated with increased risk of B-cell lymphoma subtypes. Hum Mol Genet 2016;25:1663-76. [Crossref] [PubMed]
- Widmann TA, Herrmann M, Taha N, et al. Short telomeres in aggressive non-Hodgkin's lymphoma as a risk factor in lymphomagenesis. Exp Hematol 2007;35:939-46. [Crossref] [PubMed]
- Hosnijeh FS, Matullo G, Russo A, et al. Prediagnostic telomere length and risk of B-cell lymphoma-Results from the EPIC cohort study. Int J Cancer 2014;135:2910-7. [Crossref] [PubMed]
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP Alone in Elderly Patients with Diffuse Large-B-cell lymphoma. N Engl J Med 2002;346:235-42. [Crossref] [PubMed]
- Pescovitz MD. Rituximab, an Anti-CD20 Monoclonal Antibody: History and Mechanism of Action. Am J Transplant 2006;6:859-66. [Crossref] [PubMed]
- Kusumoto S, Tanaka Y, Mizokami M, et al. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol 2009;90:13-23. [Crossref] [PubMed]
- Kim SJ, Hsu C, Song YQ, et al. Hepatitis B virus reactivation in B-cell lymphoma patients treated with rituximab: analysis from the Asia Lymphoma Study Group. Eur J Cancer 2013;49:3486-96. [Crossref] [PubMed]
- Moreno C, Montillo M, Panayiotidis P, et al. Ofatumumab in poor-prognosis chronic lymphocytic leukemia: a Phase IV, non-interventional, observational study from the European Research Initiative on Chronic Lymphocytic Leukemia. Haematologica 2015;100:511-6. [Crossref] [PubMed]
- Lee HM, Liapakis A, Lim JK. Diagnosis, Management, and Prevention of Hepatitis B Reactivation. Curr Hepatol Rep 2015;14:184-94. [Crossref]
- Cheng AL, Hsiung CA, Su IJ, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology 2003;37:1320-8. [Crossref] [PubMed]
- Di Bisceglie AM, Lok AS, Martin P, et al. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: Just the tip of the iceberg? Hepatology 2015;61:703-11. [Crossref] [PubMed]
- Ogura M, Tobinai K, Hatake K, et al. Phase I study of obinutuzumab (GA101) in Japanese patients with relapsed or refractory B-cell non-Hodgkin lymphoma. Cancer Sci 2013;104:105-10. [Crossref] [PubMed]
- Cameron F, McCormack PL. Obinutuzumab: first global approval. Drugs 2014;74:147-54. [Crossref] [PubMed]
- Lau GK, Leung YH, Fong DY, et al. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood 2002;99:2324-30. [Crossref] [PubMed]
- Lok AS, Trinh H, Carosi G, et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology 2012;143:619-28.e1. [Crossref] [PubMed]
- Riedell P, Carson KR. A drug safety evaluation of rituximab and risk of hepatitis B. Expert Opin Drug Saf 2014;13:977-87. [Crossref] [PubMed]
- Iannitto E, Minardi V, Calvaruso G, et al. Hepatitis B virus reactivation and alemtuzumab therapy. Eur J Haematol 2005;74:254-8. [Crossref] [PubMed]
- Kim HY, Kim W. Chemotherapy-related reactivation of hepatitis B infection: updates in 2013. World J Gastroenterol 2014;20:14581-8. [Crossref] [PubMed]
- Pei SN, Chen CH. Risk and prophylaxis strategy of hepatitis B virus reactivation in patients with lymphoma undergoing chemotherapy with or without rituximab. Leuk Lymphoma 2015;56:1611-8. [Crossref] [PubMed]
- Wasmuth JC, Fischer HP, Sauerbruch T, et al. Fatal acute liver failure due to reactivation of hepatitis B following treatment with fludarabine/cyclophosphamide/rituximab for low grade non-Hodgkin's lymphoma. Eur J Med Res 2008;13:483-6. [PubMed]
- Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on Prevention and Treatment of Hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:221-44.e3. [Crossref] [PubMed]
- Hwang JP, Somerfield MR, Alston-Johnson DE, et al. Hepatitis B Virus Screening for Patients With Cancer Before Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update. J Clin Oncol 2015;33:2212-20. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Hodgkin’s Lymphomas. Version 4. 2014; 4. Available online: https://www.nccn.org/about/nhl.pdf
- Sandherr M, Hentrich M, von Lilienfeld-Toal M, et al. Antiviral prophylaxis in patients with solid tumours and haematological malignancies--update of the Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO). Ann Hematol 2015;94:1441-50. [Crossref] [PubMed]
- Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol 2016;22:18-75. [Crossref] [PubMed]
- Reddy KR, Beavers KL, Hammond SP, et al. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:215-9; quiz e16-7.
- Coffin CS, Fung SK, Ma MM, et al. Management of chronic hepatitis B: Canadian Association for the Study of the Liver consensus guidelines. Can J Gastroenterol 2012;26:917-38. [Crossref] [PubMed]
- European Association For the Study Of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-85. [Crossref] [PubMed]
- Koskinas JS, Deutsch M, Adamidi S, et al. The role of tenofovir in preventing and treating hepatitis B virus (HBV) reactivation in immunosuppressed patients. A real life experience from a tertiary center. Eur J Intern Med 2014;25:768-71. [Crossref] [PubMed]
- Tang CM, Yau TO, Yu J. Management of chronic hepatitis B infection: current treatment guidelines, challenges, and new developments. World J Gastroenterol 2014;20:6262-78. [Crossref] [PubMed]