MET: roles in epithelial-mesenchymal transition and cancer stemness
Introduction
Receptor tyrosine kinases (RTKs) play pivotal roles in biological processes such as embryogenesis, organogenesis, and tissue regeneration. Epidermal growth factor receptor (EGFR) and MET are among most well-studied RTKs out of 14 RTK families. Given their profound roles in cell survival, proliferation, and migration, it is not surprising that RTK activation is a common feature of cancer.
Recent advances in genomics, cellular models, molecular and chemical genetics, to name a few, significantly increased our understanding in cancer and cancer-promoting signaling pathways. Cancer is a highly dynamic and heterogeneous disease. Genomic clonal diversity, cellular hierarchy, pleiotropic signal redundancy, and tumor microenvironment simultaneously influence tumor growth and dissemination. In addition, they are critical factors to determine the efficacies of molecularly targeted therapeutic reagents.
MET and its ligand hepatocyte growth factor (HGF) (also known as scatter factor) were first identified about 30 years ago, as transforming and/or oncogenic genes (1,2). Since then, the roles of HGF-MET signaling axis have been elucidated in critical phases of tumor initiation and progression, including proliferation, survival, migration, stemness, and resistance to radiation and chemotherapy. Now, HGF-MET signaling axis is a well-known therapeutic target in various cancers and multiple therapeutic reagents have been developed for clinical application. To achieve maximal therapeutic benefit in cancer patients, it is important to understand how MET signaling drives key oncogenic programs and identify which tumors are most likely to be responsive to MET-targeted therapies.
HGF-MET signaling axis
The MET proto-oncogene is located on chromosome 7q31.2 and first identified in human osteogenic sarcoma in 1984 (1,3). MET extracellular component consists of N-terminal semaphorin (Sema), plexin-semaphorin-integrin (PSI), and immunoglobulin-plexin-transcription factor (IPT). MET intracellular component includes juxtamembrane, catalytic region, and docking site for adaptor proteins such as growth factor receptor bound protein 2 (GRB2), GRB2 association binding protein 1 (GAB1), Src homology 2 domain containing (SHC), phosphatidylinositol 3-kinase (PI3K) and others (4,5). HGF, located on chromosome 7q21.1, encodes a ligand to activate MET signaling cascade (6). HGF is a disulfide multidomain protein including N-terminal domain, four kringle domains, and C-terminal serin proteinase homology (SPH). It produces a single inactive precursor that is converted to active two-chain heterodimer linked by a disulfide bond.
Upon HGF binding, MET autophosphorylation occurs on two catalytic tyrosine residues (Tyr1234 and Try1235) within the kinase activation loop. Phosphorylation of two docking tyrosine residues near C-terminal tail (Try1349 and Try1356) forms a multifunctional docking site for signaling effectors (7). This leads to the activation of various signal transduction pathways for survival, cell growth, migration, angiogenesis, morphogenesis, and stemness. Downstream pathways that are activated by MET include PI3K-AKT, RAS-MAPK, Signal transducer and activator of transcription 3 (STAT3), nuclear factor-kappa B (NF-κB), and WNT (8-10). For example, MET activation facilitates the engagement of PI3K and GAB1 and leads to downstream signals through AKT. Then, AKT inactivates the pro-apoptotic protein BCL-2 antagonist of BAD and activates MDM2, thereby suppressing apoptosis and promoting cell survival (11). In addition, AKT also activates mammalian target of rapamycin (mTOR), which regulates cell proliferation.
GRB2-son of sevenless (GRB2-SOS) complex, formed by MET signaling, leads to activate RAS and subsequently activate RAF kinases, MAPK effector kinase (MEK), and MAPK. MAPK phosphorylates ERK to regulate cell cycle progression and cell motility (12,13). NF-κB signaling stimulates downstream gene transcription relating cell proliferation and transformation. Connection between MET and NF-κB signaling appears to be mediated by phosphorylation of either PI3K-AKT or SRC, which activates inhibitor of nuclear factor kappa B kinase (IKK) to induce degradation of IκBs and activation of NF-κB (14).
MET is known to interact with STAT3. STAT3 can directly bind to MET and induce STAT3 phosphorylation, which is necessary for cancer cell transformation and invasion, and endothelial cell proliferation and tubule morphogenesis (8,15). Similarly, MET can interacts with integrin α6β4, CD44, and plexin B1 to promote migration, invasion, and metastasis (16-18). Furthermore, MET can interact with and activate multiple RTKs. The crosstalk between MET and other RTKs including AXL, PDGFR, EGFR, ERBB2, ERBB3, RON, and VEGFR involve the regulation of organogenesis, oncogenic pathways, and resistance of targeted therapies (19-23).
Downregulation of MET signaling is achieved in a similar manner to other RTKs. Degradation of MET is initiated after ligand-dependent activation of receptor through endocytosis. The ubiquitin E3 ligase CBL induces degradation of MET by recognizing the phosphorylated Tyr1003 in the juxtamembrane domain of MET and interacting with CBL-interacting protein 85 (24,25). Another mechanism of MET downregulation is through the regulated proteolysis mediated by a disintegrin and metalloprotease (ADAM) like protease. The intracellular domain undergoes proteolysis by γ-secretase and subsequently degrades by proteasome (26).
Physiological roles of MET signaling
During normal development, the interaction between MET (expressed in epithelial cells and myoblast progenitors) and HGF (secreted from mesenchymal cells) is tightly regulated and plays central roles in organogenesis. During embryogenesis, MET promotes survival and proliferation of hepatocytes and labyrinth trophoblast progenitor cells to generate the placental labyrinth (27). MET knockout impeded placental and liver development leading to death of the animal, indicating critical roles of MET in these developmental programs (28). MET controls epithelial-mesenchymal transition (EMT) of long range migrating myogenic progenitor cells during hypaxial muscle development (29). Moreover, MET is responsible for the development of sensory neuron to induce survival and differentiation from progenitor cells and axon outgrowth (30). In adult, MET regulates organ regeneration in liver, skin, and kidney upon acute or chronic damage (31-33). MET-expressing bone marrow stem cells migrate to and involve in the repair processed in the injured tissues such as liver, skeletal muscle, and damaged neuronal tissue. Consistent with this, HGF levels are increased in patient stroma after hepatectomy and renal transplantation (34,35). Conditional MET knockout in mice hepatocytes inhibited cell cycle progression and liver regeneration after hepatectomy (31,36). In addition, MET is essential for proliferation and correct orientation of the keratinocytes during skin wound repair (32). These data collectively suggest that MET signaling promotes EMT process during embryonic development and cell survival during the life.
MET signaling in cancer
Aberrantly activated MET pathway contributes to tumor progression, invasion, metastasis, and recurrence by promoting tumor cell survival, proliferation, migration, EMT, angiogenesis, treatment resistance, and maintenance of stemness.
Proliferation and survival
Activation of MET enhances tumor cell proliferation in gliomas (37), lung cancer (38), and head and neck squamous cell carcinomas (HNSCCs) (39) through activation of downstream cascades such as c-Myc and STAT3. Overexpression of HGF or MET enhance tumor growth in animal models (40,41), while short hairpin RNA (shRNA) mediated MET knockdown significantly suppressed tumor growth (42). HGF/MET signaling inhibits tumor cell apoptosis induced by chemotherapy and irradiation. For example, MET activation by HGF was shown to significantly decrease cisplatin, taxol, and gamma irradiation-induced cell death in glioblastoma (43).
Migration, invasion and metastasis
Similar to developmental processes, HGF-MET signaling axis stimulates cancer cell motility (40,41). As shown in MET knockdown in vivo model of HNSCC, MET inhibition induced the decreased cell motility, lymph node metastasis, and subsequent prolongation of animal survival (44). HGF increases invasiveness through matrix metalloproteinase-2 (MMP-2) and urokinase-type plasminogen activator (uPA) expression and activation. Furthermore, high levels of MET and Snail, a key regulator of EMT, correlate with highly invasive tumor phenotypes and portend poor prognosis in basal breast cancer (45). MET induces metastasis in different organs through RAS-MAPK, RAC1, and WNT activity in cancer (10,46).
Angiogenesis and stromal cell communication
MET induces tumor angiogenesis through stimulation of endothelial cell proliferation, migration, and tubulogenesis (47,48). MET mRNA expression is positively regulated by hypoxia inducible factor 1α (HIF1α), an oxygen sensor in several types of cancers (48-50). MET signaling induces the expression of vascular endothelial growth factor A (VEGFA) by interaction of SHCs and reduces thrombospondin 1 (TSP1), the angiogenesis suppressor (23). MET and VEGFR could not transphosphorylate each other, but share common signaling molecules including MAPK, ERK, AKT, and FAK (51). Activation of MET in endothelia cells enhances proliferation, migration, elongation in collagen gels and angiogenesis in a matrigel plug assay (47,52). In contrast, MET inhibitor (decoy MET) reduces tumor growth via in part by inhibition of angiogenesis (47).
MET pathway also regulates bone marrow derived cells in tumor progression. Activation of MET-STAT3 signaling induces myeloid derived suppressor cells to suppress immune system in cancer by HGF secretion in mesenchymal stem and progenitor cells. Myeloid derived suppressor cells express inducible nitric oxide synthase (iNOS) and arginase 1 to activate regulatory T cells (53). HGF/MET signaling also suppresses dendritic cells antigen presenting ability, Th1 and Th2 immune responses, and induces anti-inflammatory cytokines (54). HGF induces secretion of chemokines (MIP-1β, MIP-2α), interleukins (IL-6, IL-8, IL-10), and iNOS in monocytes (55,56) and upregulates NF-κB signals in macrophages to induce anti-inflammatory roles (57).
Cancer stem cells (CSCs)
CSCs are a subpopulation of highly tumorigenic cells that harbor stem cell characteristics. While our understanding of CSCs is still evolving, studies from multiple groups support the model that CSCs drive GBM propagation and foster resistance to conventional therapies such as radiation and chemotherapy (58-60). HGF/MET signaling is essential for CSC maintenance in several cancers including colorectal (61), breast (62), prostate (63), pancreatic cancer (64), and glioblastoma (65). In colorectal cancer, MET activates WNT-β-catenin signaling cascade to promote stemness and invasion (10,66). Moreover, HGF increases β-catenin activity through phosphorylation of β-catenin and interaction with BCL9L, which enhances β-catenin transcriptional activity to regulate migration and invasion (67). During breast cancer metastasis to bone, bone derived HGF activates MET-WNT-β-catenin signals to maintain stem cell properties (68). In mouse model of basal-like breast cancer, constitutive activation of MET suppresses differentiation of mammary luminal progenitor cells and induces stem cell characteristics (69). In prostate and pancreatic cancer, both HGF and MET are reported to be preferentially expressed in stem-like tumor cells (70,71). While preferential expression of MET in stem-like cells in various cancer, it is unclear whether HGF expression has similar pattern. In glioblastoma, MET activation promotes stemness and induces invasive phenotype (72). Several groups have shown that MET is preferentially expressed in glioblastoma stem cells (GSCs) and confer radio-and chemo resistance to GSCs. Using xenograft models, MET inhibitors (anti-HGF, anti-MET, and MET targeted small molecule inhibitors) decrease tumor progression and the expression of stem markers such as, CD133, Sox2, and Nanog (73). Thus, these studies collectively support the roles of MET signaling in CSC maintenance.
Treatment resistance
Chemotherapy and radiation therapy are considered as standard-of-care for cancer patients. While these treatments exert cytotoxic effects and reduce tumor burden, however, tumors often acquire resistance and eventually recur. MET signaling sustains resistance mechanism by promoting invasive growth program and/or EMT-like properties and protecting cells from apoptosis. Cisplatin treatment induces MET expression in HNSCC during metastasis (74). Taxanes increases MET expression through suppression of MET targeted miR-31 in ovarian cancer (75). The expression of MET is increased after radiation by ATM kinases and NF-κB to protect from DNA damage agents (76). During cell response to DNA damage, loss of functional mutations in p53 leads to activate MET through accumulation of MET mRNA by suppression of miR-34 and activation of Sp-1 (77); stimulation of MET protein endocytosis by Rab-dependent receptor recycling (78).
MET signaling is one of major resistance mechanisms to targeted therapies. Most prominent examples include MET dependent resistance in EGFR inhibitor therapies. In lung tumors in which tumors responded to EGFR inhibitors initially but became resistant, MET hyperactivation has been found in tumors. Overexpression of MET can bypass EGFR and activate PI3K-AKT signals in these tumors (79,80). Moreover, HGF induces resistance to EGFR therapies through recruitment Axl and EphA2 in EGFR complex. Co-treatment with anti-MET and EGFR inhibitors significantly suppress tumor growth and recurrence (81), suggesting a potential combinatory therapeutic approach. Crosstalk between MET and other RTKs including Axl, EGFR, ERBB2, ERBB3, RON, and IGF1R is known to be responsible for resistance to other targeted therapies (20). A major resistance mechanism against anti-VEGFR therapies in glioblastoma involves MET signaling (82). In bevacizumab resistant tumors, MET was induced by hypoxia and promoted tumor cell invasion and survival. Suppression of MET in bevacizumab resistant glioma models impeded tumor invasion and prolonged animal survival (83). In melanoma, MET contributes to resistance to the BRAF inhibitor, vemurafenib via activation of ERK-MAPK and PI3K-AKT (84). Collectively, MET signaling is a key resistance mechanism against therapies and need to be targeted to overcome tumor resistance.
Genetic abnormalities in cancer
Aberrantly activated or deregulated HGF/MET axis has been found in several human tumor types including liver, lung, brain, breast, bladder, colorectal, and gastric cancer and contributes to tumor progression, survival, and invasion (85,86). Hyperactivation of HGF/MET signaling occurs by chromosomal rearrangement (1), chromosome and/or focal amplification (87), activating mutations (88), increased ligand expression (89), and alterations in other pathways (77,90). MET was discovered as TPR-MET oncogene [translocated promoter region (tpr) and MET kinase domain] in human osteosarcoma (91) and gastric cancer (92). Recently, MET fusion proteins (CLIP2-MET and PTPRZ1-MET) were identified in pediatric glioblastoma up to 10% of cases (93). MET gene amplification has been identified in non-small cell lung cancer (NSCLC), HNSCC, breast, colorectal, gastric cancer, and glioblastoma (94). In NSCLC, MET amplification is positively correlated with poor prognosis (87). In colorectal cancer, MET amplification significantly increased invasive phenotype during tumor progression and metastasis (90). In gastric cancer, MET amplification portends poor patient survival. The frequencies of MET amplification range from 2% to 24% in gastric cancer (87,95). Compared to the frequencies of MET gene amplification, activating MET mutations appear to be relatively rare in solid tumors. The first identified missense mutations in kinase domain (M1268T, Y1235D, and Y1230C/H/D) are related to development of hereditary papillary renal cell carcinoma (RCC) (88). Kinase domain mutations in MET protein lead to constitutively active kinases and increase accumulation of MET through avoidance of lysosomal degradation (96). Mutation in the binding site of CBL enhances MET phosphorylation in NSCLC and melanoma (97). HGF levels are often elevated in cancers and stroma in NSCLC, HNSCC, gastric, brain, and breast cancer via upregulation of MET signaling. HGF is also co-expressed with MET in cancer to drive invasive phenotypes and metastasis in breast cancer and acute myeloid leukemia (AML) (98-100). Independent of genomic alterations, hyperactivation of MET in cancer can be achieved by the increased MET mRNA level. For example, MET expression is induced by hypoxia (HIF1α mediated transcription) (50,101) and inflammatory cytokines (interleukin-6 and tumor necrosis factor-α) (102,103), tumor suppressor p53 (77), and MET targeted microRNAs (miR-1 and miR-34) (90).
MET-targeted therapies
HGF and MET targeted inhibitors have been developed and tested in preclinical and clinical trials in numerous tumors (104-106). These reagents include blocking antibodies against HGF or MET, and small molecule inhibitors that could target the active site of MET to suppress phosphorylation or the interaction between MET and downstream signaling effectors.
Anti-HGF antibodies: ficlatuzumab and rilotumumab
Ficlatuzumab is a humanized HGF monoclonal antibody under used in a phase II investigation of Asian lung adenocarcinoma patients (104). Patients were treated with ficlatuzumab in combination with gefitinib (an EGFR inhibitor), or gefitinib alone. The treatment groups did not show significant differences in overall survival (OS) and progression-free survival (PFS), but high HGF-expressing subgroup showed the prolonged OS and PFS with combination therapies. This result suggests that HGF inhibition can suppress resistance to the EGFR therapy and subsequent tumor progression. Ongoing phase II study is planned to compare ficlatuzumab plus erlotinib to erlotinib alone in NSCLC patients whom were selected for EGFR mutational status. Rilotumumab is another HGF targeted human monoclonal antibody and has been studied in phase II trials in gastric cancer or gastroesophageal junction adenocarcinoma under treated with cytotoxic agents such as cisplatin, epirubicin, and capecitabine. MET-positive patients showed the increased OS and PFS in rilotumumab plus chemotherapy (105). However, phase II studies in recurrent glioblastoma patients and castration resistant prostate cancer patients did not show significant effects for rilotumumab (106).
Anti-MET antibodies: onartuzumab (MetMAB)
Onartuzumab is a humanized monoclonal antibody that directly binds to the Sema domain, the ligand binding site of MET, and inhibits the interaction between HGF and MET. In NSCLC, a phase II trial compared onartuzumab plus erlotinib with erlotinib alone. Significant improvements of OS and PFS were observed in MET positive subgroup (107,108). Onartuzumab is also being tested in a phase III study in metastatic HER2-negative, MET positive gastroesophageal cancer, and phase II study in metastatic colorectal cancer and glioblastoma (109).
Small molecule inhibitors: crizotinib, cabozantinib, foretinib, tivantinib
Crizotinib is a multi-kinase inhibitor including MET, anaplastic lymphoma kinase (ALK) and ROS1. This drug has been approved for ALK positive NSCLC patients. Crizotinib significantly increased PFS and OS in NSCLC patients (110). Moreover, crizotinib could inhibit MET in preclinical studies and showed high efficacy in MET-overexpressing NSCLC tumors in phase I and II trials (111).
Cabozantinib is an orally available multi-kinase inhibitor targeting MET, VEGFR2, RET, KIT, and FLT3 and it was approved for treatment of progressive, metastatic medullary thyroid carcinoma (MTC) (112). Foretinib is an oral multi-kinase inhibitor targeted MET, RON, AXL, TIE2, and VEGFR and tested clinical efficacy in metastatic gastric cancer (113). In advanced papillary RCC, foretinib showed efficacy in overall response rate (13.5%) (114). Tivantinib is a non-ATP competitive inhibitor for MET. Phase II trial for tivantinib monotherapy increased PFS but could not improve OS in HCC patients (115). A phase III trial of tivantinib plus erlotinib and erlotinib alone in EGFR tyrosine kinase inhibitor-naive NSCLC patients showed limited therapeutic responses (116).
Conclusions
MET signaling promotes various oncogenic programs during tumor initiation, progression, metastasis, and treatment resistance. Accordingly, various MET targeting approaches have been developed and some of which are in advanced stages of clinical trials (Figure 1). There are several critical factors to be considered for the maximal therapeutic benefit of MET-targeted therapy.
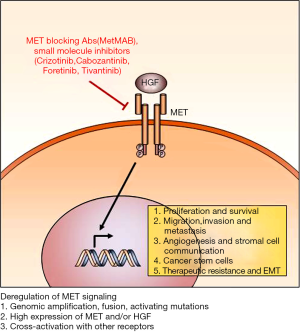
It is increasingly clear that genetic complexity, inter- and intra-tumoral heterogeneity, and molecular adaptability of cancer can determine therapeutic responses of molecularly targeted therapies. A clear discordance between profound anti-tumor effects in preclinical studies and clinical benefit in human patients has been frequently observed. While there are multiple reasons behind this discrepancy, stratification of the patients who are most likely to respond to MET-targeted therapies may significantly improve clinical outcomes. The patient subgroup with genomic MET alterations is one of most obvious choices. Compared to the patients with EGFR alterations, patients with genomic MET alterations were relatively infrequent. Recent advances in genomic screening may facilitate pre-selection of these patient groups. For example, whole genome sequencing of pediatric glioblastoma recently identified the patient subgroup with oncogenic MET fusion and crizotinib showed strong anti-tumor effects in this subgroup (93).
As learned from EGFR-targeted therapies, de novo mutations or expansion of minor resistant clones can emerge from MET-targeted therapies. Through analysis of patient specimens before and after treatment will be important to understand the potential resistance mechanisms and identify the biomarkers that are associated with therapeutic responses. Currently, biomarkers used in MET-targeted therapies are rather limited to the expression levels of HGF and/or MET. Additional molecular markers that predict the MET pathway dependency of the tumor or therapeutic effects of MET targeting are urgently required.
Hyperactive MET signaling in CSC subpopulation and EMT process may occur independent of genomic alterations. MET signaling is a crucial regulator of EMT and stem cell renewal in early embryonic development and adult stem cells. The roles of MET in stem cell renewal and EMT may share common downstream signals because EMT has been linked to acquisition of stem cell status (117). As MET pathway activation has been implicated in treatment resistance mechanisms including irradiation and EGFR-targeted therapies, combination therapies involving MET-targeting should be attractive therapeutic approaches. One of potential concerns in MET-targeted therapies is systemic toxicity of the drug. As MET signaling is implicated in normal stem cell maintenance, tissue repair and hematopoiesis, treatment of MET inhibitors may induce unwanted side effects including myelosuppression and mucosal injury and wound healing complications (7). Indeed, peripheral edema has been associated in multiple tumor types after MET inhibitor trails, because of the suppression of MET signaling in vascular endothelial cells (118). Localized delivery of anticancer drugs may offer advantages by decreasing systemic side effects and achieving a high intra-tumoral drug concentration (119).
Challenges inherent in developing effective cancer therapeutics include genetic complexity, inter- and intra-tumoral heterogeneity, molecular adaptability of tumor, CSC, and treatment resistance. MET-targeted therapies have a potential to become an effective and sustainable anti-cancer approaches.
Acknowledgements
Funding: This work was supported by NIH grants R01 NS082312 and R01 NS083767 (J Lee).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cooper CS, Park M, Blair DG, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984;311:29-33. [Crossref] [PubMed]
- Giordano S, Ponzetto C, Di Renzo MF, et al. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature 1989;339:155-6. [Crossref] [PubMed]
- Park M, Dean M, Kaul K, et al. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci U S A 1987;84:6379-83. [Crossref] [PubMed]
- Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 1994;77:261-71. [Crossref] [PubMed]
- Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene 2000;19:5582-9. [Crossref] [PubMed]
- Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991;251:802-4. [Crossref] [PubMed]
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010;11:834-48. [Crossref] [PubMed]
- Syed ZA, Yin W, Hughes K, et al. HGF/c-met/Stat3 signaling during skin tumor cell invasion: indications for a positive feedback loop. BMC Cancer 2011;11:180. [Crossref] [PubMed]
- Müller M, Morotti A, Ponzetto C. Activation of NF-kappaB is essential for hepatocyte growth factor-mediated proliferation and tubulogenesis. Mol Cell Biol 2002;22:1060-72. [Crossref] [PubMed]
- Vermeulen L, De Sousa EM, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 2010;12:468-76. [Crossref] [PubMed]
- Xiao GH, Jeffers M, Bellacosa A, et al. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A 2001;98:247-52. [Crossref] [PubMed]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007;26:3291-310. [Crossref] [PubMed]
- Paumelle R, Tulasne D, Kherrouche Z, et al. Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene 2002;21:2309-19. [Crossref] [PubMed]
- Fan S, Gao M, Meng Q, et al. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene 2005;24:1749-66. [Crossref] [PubMed]
- Boccaccio C, Ando M, Tamagnone L, et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 1998;391:285-8. [Crossref] [PubMed]
- Chen SY, Chen HC. Direct interaction of focal adhesion kinase (FAK) with Met is required for FAK to promote hepatocyte growth factor-induced cell invasion. Mol Cell Biol 2006;26:5155-67. [Crossref] [PubMed]
- Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol 2009;19:542-51. [Crossref] [PubMed]
- Giordano S, Corso S, Conrotto P, et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 2002;4:720-4. [Crossref] [PubMed]
- Yeh CY, Shin SM, Yeh HH, et al. Transcriptional activation of the Axl and PDGFR-alpha by c-Met through a ras- and Src-independent mechanism in human bladder cancer. BMC Cancer 2011;11:139. [Crossref] [PubMed]
- Gusenbauer S, Vlaicu P, Ullrich A. HGF induces novel EGFR functions involved in resistance formation to tyrosine kinase inhibitors. Oncogene 2013;32:3846-56. [Crossref] [PubMed]
- Khoury H, Naujokas MA, Zuo D, et al. HGF converts ErbB2/Neu epithelial morphogenesis to cell invasion. Mol Biol Cell 2005;16:550-61. [Crossref] [PubMed]
- Follenzi A, Bakovic S, Gual P, et al. Cross-talk between the proto-oncogenes Met and Ron. Oncogene 2000;19:3041-9. [Crossref] [PubMed]
- Zhang YW, Su Y, Volpert OV, et al. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci U S A 2003;100:12718-23. [Crossref] [PubMed]
- Peschard P, Fournier TM, Lamorte L, et al. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol Cell 2001;8:995-1004. [Crossref] [PubMed]
- Petrelli A, Gilestro GF, Lanzardo S, et al. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature 2002;416:187-90. [Crossref] [PubMed]
- Foveau B, Ancot F, Leroy C, et al. Down-regulation of the met receptor tyrosine kinase by presenilin-dependent regulated intramembrane proteolysis. Mol Biol Cell 2009;20:2495-507. [Crossref] [PubMed]
- Schmidt C, Bladt F, Goedecke S, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995;373:699-702. [Crossref] [PubMed]
- Uehara Y, Minowa O, Mori C, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995;373:702-5. [Crossref] [PubMed]
- Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol 1998;8:404-10. [Crossref] [PubMed]
- Maina F, Hilton MC, Ponzetto C, et al. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev 1997;11:3341-50. [Crossref] [PubMed]
- Huh CG, Factor VM, Sanchez A, et al. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A 2004;101:4477-82. [Crossref] [PubMed]
- Chmielowiec J, Borowiak M, Morkel M, et al. c-Met is essential for wound healing in the skin. J Cell Biol 2007;177:151-62. [Crossref] [PubMed]
- Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol 2004;287:F7-16. [Crossref] [PubMed]
- Tomiya T, Tani M, Yamada S, et al. Serum hepatocyte growth factor levels in hepatectomized and nonhepatectomized surgical patients. Gastroenterology 1992;103:1621-4. [Crossref] [PubMed]
- Takada S, Namiki M, Takahara S, et al. Serum HGF levels in acute renal rejection after living related renal transplantation. Transpl Int 1996;9:151-4. [Crossref] [PubMed]
- Borowiak M, Garratt AN, Wustefeld T, et al. Met provides essential signals for liver regeneration. Proc Natl Acad Sci U S A 2004;101:10608-13. [Crossref] [PubMed]
- Li Y, Lal B, Kwon S, et al. The scatter factor/hepatocyte growth factor: c-met pathway in human embryonal central nervous system tumor malignancy. Cancer Res 2005;65:9355-62. [Crossref] [PubMed]
- Krishnaswamy S, Kanteti R, Duke-Cohan JS, et al. Ethnic differences and functional analysis of MET mutations in lung cancer. Clin Cancer Res 2009;15:5714-23. [Crossref] [PubMed]
- Knowles LM, Stabile LP, Egloff AM, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res 2009;15:3740-50. [Crossref] [PubMed]
- Rong S, Segal S, Anver M, et al. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci U S A 1994;91:4731-5. [Crossref] [PubMed]
- Takayama H, LaRochelle WJ, Sharp R, et al. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A 1997;94:701-6. [Crossref] [PubMed]
- Taulli R, Scuoppo C, Bersani F, et al. Validation of met as a therapeutic target in alveolar and embryonal rhabdomyosarcoma. Cancer Res 2006;66:4742-9. [Crossref] [PubMed]
- Bowers DC, Fan S, Walter KA, et al. Scatter factor/hepatocyte growth factor protects against cytotoxic death in human glioblastoma via phosphatidylinositol 3-kinase- and AKT-dependent pathways. Cancer Res 2000;60:4277-83. [PubMed]
- Tao X, Hill KS, Gaziova I, et al. Silencing Met receptor tyrosine kinase signaling decreased oral tumor growth and increased survival of nude mice. Oral Oncol 2014;50:104-12. [Crossref] [PubMed]
- Ponzo MG, Lesurf R, Petkiewicz S, et al. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc Natl Acad Sci U S A 2009;106:12903-8. [Crossref] [PubMed]
- Webb CP, Taylor GA, Jeffers M, et al. Evidence for a role of Met-HGF/SF during Ras-mediated tumorigenesis/metastasis. Oncogene 1998;17:2019-25. [Crossref] [PubMed]
- Michieli P, Mazzone M, Basilico C, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell 2004;6:61-73. [Crossref] [PubMed]
- Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro Oncol 2005;7:436-51. [Crossref] [PubMed]
- Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003;3:347-61. [Crossref] [PubMed]
- Hara S, Nakashiro K, Klosek SK, et al. Hypoxia enhances c-Met/HGF receptor expression and signaling by activating HIF-1alpha in human salivary gland cancer cells. Oral Oncol 2006;42:593-8. [Crossref] [PubMed]
- Sulpice E, Ding S, Muscatelli-Groux B, et al. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell 2009;101:525-39. [Crossref] [PubMed]
- Silvagno F, Follenzi A, Arese M, et al. In vivo activation of met tyrosine kinase by heterodimeric hepatocyte growth factor molecule promotes angiogenesis. Arterioscler Thromb Vasc Biol 1995;15:1857-65. [Crossref] [PubMed]
- Yen BL, Yen ML, Hsu PJ, et al. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Reports 2013;1:139-51. [Crossref] [PubMed]
- Okunishi K, Dohi M, Nakagome K, et al. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol 2005;175:4745-53. [Crossref] [PubMed]
- Beilmann M, Vande Woude GF, Dienes HP, et al. Hepatocyte growth factor-stimulated invasiveness of monocytes. Blood 2000;95:3964-9. [PubMed]
- Galimi F, Cottone E, Vigna E, et al. Hepatocyte growth factor is a regulator of monocyte-macrophage function. J Immunol 2001;166:1241-7. [Crossref] [PubMed]
- Coudriet GM, He J, Trucco M, et al. Hepatocyte growth factor modulates interleukin-6 production in bone marrow derived macrophages: implications for inflammatory mediated diseases. PLoS One 2010;5:e15384. [Crossref] [PubMed]
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756-60. [Crossref] [PubMed]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5:275-84. [Crossref] [PubMed]
- Das S, Srikanth M, Kessler JA. Cancer stem cells and glioma. Nat Clin Pract Neurol 2008;4:427-35. [Crossref] [PubMed]
- O'Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445:106-10. [Crossref] [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [Crossref] [PubMed]
- Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005;65:10946-51. [Crossref] [PubMed]
- Wang Z, Ali S, Banerjee S, et al. Activated K-Ras and INK4a/Arf deficiency promote aggressiveness of pancreatic cancer by induction of EMT consistent with cancer stem cell phenotype. J Cell Physiol 2013;228:556-62. [Crossref] [PubMed]
- Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 2004;64:7011-21. [Crossref] [PubMed]
- Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009;457:608-11. [Crossref] [PubMed]
- Brembeck FH, Schwarz-Romond T, Bakkers J, et al. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev 2004;18:2225-30. [Crossref] [PubMed]
- Previdi S, Maroni P, Matteucci E, et al. Interaction between human-breast cancer metastasis and bone microenvironment through activated hepatocyte growth factor/Met and beta-catenin/Wnt pathways. Eur J Cancer 2010;46:1679-91. [Crossref] [PubMed]
- Gastaldi S, Sassi F, Accornero P, et al. Met signaling regulates growth, repopulating potential and basal cell-fate commitment of mammary luminal progenitors: implications for basal-like breast cancer. Oncogene 2013;32:1428-40. [Crossref] [PubMed]
- van Leenders GJ, Sookhlall R, Teubel WJ, et al. Activation of c-MET induces a stem-like phenotype in human prostate cancer. PLoS One 2011;6:e26753. [Crossref] [PubMed]
- Li C, Wu JJ, Hynes M, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 2011;141:2218-2227.e5. [Crossref] [PubMed]
- Joo KM, Jin J, Kim E, et al. MET signaling regulates glioblastoma stem cells. Cancer Res 2012;72:3828-38. [Crossref] [PubMed]
- Rath P, Lal B, Ajala O, et al. In Vivo c-Met Pathway Inhibition Depletes Human Glioma Xenografts of Tumor-Propagating Stem-Like Cells. Transl Oncol 2013;6:104-11. [Crossref] [PubMed]
- Sun S, Wang Z. Head neck squamous cell carcinoma c-Met(+) cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer 2011;129:2337-48. [Crossref] [PubMed]
- Mitamura T, Watari H, Wang L, et al. Downregulation of miRNA-31 induces taxane resistance in ovarian cancer cells through increase of receptor tyrosine kinase MET. Oncogenesis 2013;2:e40. [Crossref] [PubMed]
- De Bacco F, D'Ambrosio A, Casanova E, et al. MET inhibition overcomes radiation resistance of glioblastoma stem-like cells. EMBO Mol Med 2016;8:550-68. [Crossref] [PubMed]
- Hwang CI, Matoso A, Corney DC, et al. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc Natl Acad Sci U S A 2011;108:14240-5. [Crossref] [PubMed]
- Muller PA, Trinidad AG, Timpson P, et al. Mutant p53 enhances MET trafficking and signalling to drive cell scattering and invasion. Oncogene 2013;32:1252-65. [Crossref] [PubMed]
- Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007;104:20932-7. [Crossref] [PubMed]
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43. [Crossref] [PubMed]
- Wang W, Li Q, Takeuchi S, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancer. Clin Cancer Res 2012;18:1663-71. [Crossref] [PubMed]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008;8:592-603. [Crossref] [PubMed]
- Jahangiri A, De Lay M, Miller LM, et al. Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res 2013;19:1773-83. [Crossref] [PubMed]
- Qin Y, Roszik J, Chattopadhyay C, et al. Hypoxia-Driven Mechanism of Vemurafenib Resistance in Melanoma. Mol Cancer Ther 2016;15:2442-54. [Crossref] [PubMed]
- Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003;4:915-25. [Crossref] [PubMed]
- Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12:89-103. [Crossref] [PubMed]
- Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A 2006;103:2316-21. [Crossref] [PubMed]
- Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 1997;16:68-73. [Crossref] [PubMed]
- Koochekpour S, Jeffers M, Rulong S, et al. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res 1997;57:5391-8. [PubMed]
- Migliore C, Martin V, Leoni VP, et al. MiR-1 downregulation cooperates with MACC1 in promoting MET overexpression in human colon cancer. Clin Cancer Res 2012;18:737-47. [Crossref] [PubMed]
- Soman NR, Wogan GN, Rhim JS. TPR-MET oncogenic rearrangement: detection by polymerase chain reaction amplification of the transcript and expression in human tumor cell lines. Proc Natl Acad Sci U S A 1990;87:738-42. [Crossref] [PubMed]
- Soman NR, Correa P, Ruiz BA, et al. The TPR-MET oncogenic rearrangement is present and expressed in human gastric carcinoma and precursor lesions. Proc Natl Acad Sci U S A 1991;88:4892-6. [Crossref] [PubMed]
- International Cancer Genome Consortium PedBrain Tumor Project. Recurrent MET fusion genes represent a drug target in pediatric glioblastoma. Nat Med 2016;22:1314-20. [Crossref] [PubMed]
- Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol 2012;9:314-26. [Crossref] [PubMed]
- Kawakami H, Okamoto I, Arao T, et al. MET amplification as a potential therapeutic target in gastric cancer. Oncotarget 2013;4:9-17. [PubMed]
- Michieli P, Basilico C, Pennacchietti S, et al. Mutant Met-mediated transformation is ligand-dependent and can be inhibited by HGF antagonists. Oncogene 1999;18:5221-31. [Crossref] [PubMed]
- Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res 2005;65:1479-88. [Crossref] [PubMed]
- Kentsis A, Reed C, Rice KL, et al. Autocrine activation of the MET receptor tyrosine kinase in acute myeloid leukemia. Nat Med 2012;18:1118-22. [Crossref] [PubMed]
- Jin L, Fuchs A, Schnitt SJ, et al. Expression of scatter factor and c-met receptor in benign and malignant breast tissue. Cancer 1997;79:749-60. [Crossref] [PubMed]
- Navab R, Liu J, Seiden-Long I, et al. Co-overexpression of Met and hepatocyte growth factor promotes systemic metastasis in NCI-H460 non-small cell lung carcinoma cells. Neoplasia 2009;11:1292-300. [Crossref] [PubMed]
- Ide T, Kitajima Y, Miyoshi A, et al. Tumor-stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer cells through the hepatocyte growth factor/c-Met pathway. Int J Cancer 2006;119:2750-9. [Crossref] [PubMed]
- Zhu X, Humphrey PA. Overexpression and regulation of expression of scatter factor/hepatocyte growth factor in prostatic carcinoma. Urology 2000;56:1071-4. [Crossref] [PubMed]
- Torti D, Sassi F, Galimi F, et al. A preclinical algorithm of soluble surrogate biomarkers that correlate with therapeutic inhibition of the MET oncogene in gastric tumors. Int J Cancer 2012;130:1357-66. [Crossref] [PubMed]
- Mok TS, Geater SL, Su WC, et al. A Randomized Phase 2 Study Comparing the Combination of Ficlatuzumab and Gefitinib with Gefitinib Alone in Asian Patients with Advanced Stage Pulmonary Adenocarcinoma. J Thorac Oncol 2016;11:1736-44. [Crossref] [PubMed]
- Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol 2014;15:1007-18. [Crossref] [PubMed]
- Wen PY, Schiff D, Cloughesy TF, et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol 2011;13:437-46. [Crossref] [PubMed]
- Spigel DR, Edelman MJ, Mok T, et al. Treatment Rationale Study Design for the MetLung Trial: A Randomized, Double-Blind Phase III Study of Onartuzumab (MetMAb) in Combination With Erlotinib Versus Erlotinib Alone in Patients Who Have Received Standard Chemotherapy for Stage IIIB or IV Met-Positive Non-Small-Cell Lung Cancer. Clin Lung Cancer 2012;13:500-4. [Crossref] [PubMed]
- Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2013;31:4105-14. [Crossref] [PubMed]
- Bendell JC, Ervin TJ, Gallinson D, et al. Treatment rationale and study design for a randomized, double-blind, placebo-controlled phase II study evaluating onartuzumab (MetMAb) in combination with bevacizumab plus mFOLFOX-6 in patients with previously untreated metastatic colorectal cancer. Clin Colorectal Cancer 2013;12:218-22. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Blackhall F, Kim DW, Besse B, et al. Patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. J Thorac Oncol 2014;9:1625-33. [Crossref] [PubMed]
- Cabanillas ME, Brose MS, Holland J, et al. A phase I study of cabozantinib (XL184) in patients with differentiated thyroid cancer. Thyroid 2014;24:1508-14. [Crossref] [PubMed]
- Shah MA, Wainberg ZA, Catenacci DV, et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS One 2013;8:e54014. [Crossref] [PubMed]
- Choueiri TK, Vaishampayan U, Rosenberg JE, et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol 2013;31:181-6. [Crossref] [PubMed]
- Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol 2013;14:55-63. [Crossref] [PubMed]
- Scagliotti G, von Pawel J, Novello S, et al. Phase III Multinational, Randomized, Double-Blind, Placebo-Controlled Study of Tivantinib (ARQ 197) Plus Erlotinib Versus Erlotinib Alone in Previously Treated Patients With Locally Advanced or Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2667-74. [Crossref] [PubMed]
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15. [Crossref] [PubMed]
- Maroun CR, Rowlands T. The Met receptor tyrosine kinase: a key player in oncogenesis and drug resistance. Pharmacol Ther 2014;142:316-38. [Crossref] [PubMed]
- Jain V, Jain S, Mahajan SC. Nanomedicines based drug delivery systems for anti-cancer targeting and treatment. Curr Drug Deliv 2015;12:177-91. [Crossref] [PubMed]


