Gastric metastases from gynaecologic tumors: case reports and review of the literature
Introduction
The stomach is an infrequent localization of tumor metastases. In three large autopsy series an incidence rate as low as 0.54–1.7% has been found (1-3). An even lower frequency (0.2%) has been reported at clinical-endoscopic studies (4). It has been stated that gastric metastases predominantly arise from malignant melanoma, followed by lung and breast cancer (3,5). On the contrary, both ovarian and uterine metastases in the stomach are extremely infrequent. Clinical and endoscopic features of gastric metastases are nonspecific and this, together with their low frequency, contributes to a scarce knowledge among endoscopists on such a clinically relevant entity. Moreover, it has been found that even the biopsy sampling of endoscopically detected gastric lesions may be unsuccessful for an appropriate histological definition, gastric metastases no rarely sparing the mucosal layer. In addition, some metastatic tumors may mimicry a primary gastric cancer, at both endoscopic and histological assessment. For instance, invasive lobular carcinoma of the breast may resemblance to gastric signet ring cell carcinoma (6). It is intuitive that a correct distinction between primary and metastatic gastric carcinoma is extremely important because of the therapeutic approach completely differs. Here, we described the case of three females with either ovarian, uterine, or breast metastases in the stomach observed in a single centre, and we performed a systematic review of the literature.
Case series
Ovarian tumor metastasis
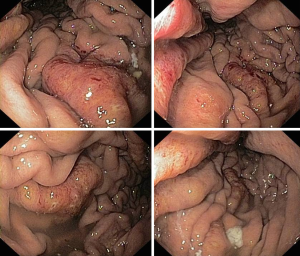
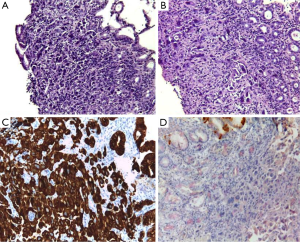
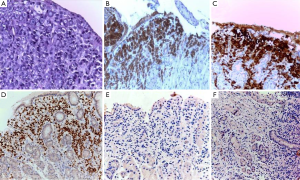
A 49-year-old woman was diagnosed with left-side serous ovarian adenocarcinoma on 2012 when she underwent surgical hysterectomy, with bilateral salpingo-oophorectomy and pelvic lymph node dissection. In addition, adjuvant chemotherapy was administered. The patient was in good condition until July 2014 when she was admitted in Department of Oncology due to abdominal pain, persisting vomiting and weight loss. At upper endoscopy, irregular, enlarged and hyperaemic folds in the gastric body were detected (Figure 1), whilst the remaining mucosa was normal until to the descending duodenum. The histological examination of multiple biopsies revealed neoplastic infiltration of gastric mucosa in accordance with a metastatic ovarian cancer (Figure 2). At this time, peritoneal carcinosis was also detected, so that the patient received a further chemotherapy, but she died within 4 months.
Uterine tumor metastasis
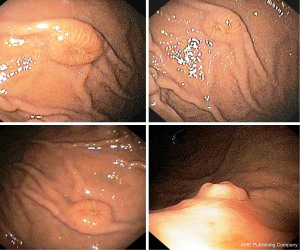
An 80-year-old woman with diabetes and hypertension underwent total hysterectomy, with bilateral salpingo-oophorectomy and pelvic lymph node dissection 10 years before due to a uterine leiomiosarcoma. She revealed a total thyroidectomy and right colectomy, both for carcinoma, 7 and 5 years before the gynaecological intervention, respectively. Following the colon cancer resection, she also received adjuvant chemotherapy. The patient was referred for upper endoscopy to our Endoscopic Unit as outpatient due to epigastric pain appeared in the last 3 months. She was receiving thyroid hormone therapy, insulin, a β-blocker, and 100 mg acetylsalicylic acid for primary cardiovascular prevention together with 30 mg lansoprazole as a gastroprotection. At upper endoscopy, multiple submucosal nodules, 7–10 mm in diameter, with apical erosion, localized in the gastric body and fundus and sparing the antral mucosa were observed (Figure 3). The histological examination disclosed neoplastic spindle cells infiltrating gastric mucosa with an immunohistochemical pattern compatible with metastatic leiomiosarcoma (Figure 4). The patient moved to another hospital so that she was lost at follow-up.

Breast tumor metastasis
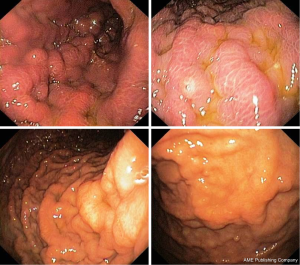
A 70-year-old woman, without relevant diseases at personal clinical history, underwent radical left mastectomy with adjuvant chemotherapy. Following 2 months from chemotherapy starting, she complained with dysphagia, particularly for liquids, and epigastric pain. Therefore, the patient undergo to upper endoscopy in another Endoscopic Unit, which showed irregular folds in the upper third of the stomach, but the histological examination of biopsies showed only inflammatory infiltration in the gastric mucosa. One month later, she was referred to our Endoscopic Unit for repeating upper endoscopy. We noted a regular oesophageal mucosa, including the cardia, which appeared easily surmountable with the standard endoscope, and we confirmed the presence of irregular fold in the gastric body mimicking linitis plastica (Figure 5). Despite several biopsies were taken, the histological examination failed to demonstrate the neoplastic nature of the lesion, revealing only reactive gastritis. Five months later, at the end of six chemotherapy cycles, a further upper endoscopy was performed due persistent epigastric pain without dysphagia. The endoscopic examination was substantially unchanged, and the histological feature eventually showed a metastatic breast cancer in the stomach (Figure 6). The patient is on ongoing chemotherapy.
Literature review
Cases of gastric metastasis from primary gynaecologic tumors—including ovarian, uterine and breast cancers—diagnosed at endoscopy and published on PubMed in English language were reviewed. All relevant articles were retrieved, and a manual search of reference lists was performed to identify any additional study that might have been missed.
On 2012, a systematic review of gastric metastasis from ovarian carcinoma was published (7), including a total of 11 case reports. However, 1 out of these case reports was erroneously included, since it was dealing with an ovarian metastasis from primary gastric cancer (8). A successive systematic review of data published between 1970 and 2013, reported a total of 9 cases (4). By selecting only different patients from the first review (10 cases) (8), the second review (3 cases) (4), and by adding further case reports published thereafter we retrieved (4 cases) (9-12), and our case report, a total of 18 patients with gastric metastases from ovarian cancer have been published. Further 3 cases were included in a study of metastases in the stomach arising from different primary tumors, but no details were specifically provided for those patients with gynaecologic metastases (3).
Overall, the mean age at metastasis diagnosis was 58.8 years (range, 41–73 years), and the median interval between the primary tumor and the diagnosis of the metastatic tumor in the stomach was 32 months (range, 14–84 months), whilst 2 patients had synchronous gastric metastases. The metastases were localized in the antrum in 13 (72%) cases and in upper two third of the stomach in remaining 5 cases. At endoscopy, the metastasis presented as submucosal mass (with or without apical erosion) in 11 (61%) cases, ulcer in 2 cases, and enlarged folds in 1 patient. The main presenting symptoms were anaemia/haemorrhage (4 cases), vomiting (2 cases), perforation (1 case), and dyspeptic symptoms (6 cases), whilst in 5 asymptomatic patients the metastasis was suspected due to either PET finding or CA-125 elevation at follow-up.
Overall, 5 studies reported cases of gastric metastasis from uterine tumors, including a case series of 4 patients, 2 series with 2 patients, and 2 single case reports (3,13-16). Therefore, by adding our case, a total of 11 patients were described. However, no specific details were provided for the 6 patients included in a retrospective study on gastric metastases of different origin (3,15), and we was unable to retrieve a publication (16). The scanty data available are summarized in Table 1.

Full table
On 2014, a systematic review on gastric metastases of different origin has been performed, including data of 4 cases series and 2 case reports on breast cancer metastases in the stomach, accounting for a total of 95 patients (4). Our literature search found further 13 publications, including 5 case series and 8 case reports, with a total of 63 patients (17-29). Therefore, by adding our case, data of 159 patients with gastric metastasis from breast cancer are available. Overall, the mean age at metastasis diagnosis extrapolated from 136 patients was 47.4 years (range, 35–90 years), and the median interval between the primary tumor and the diagnosis of the metastatic tumor in the stomach was 78 months (range, 33–96 months), whilst 8 patients had synchronous gastric metastases. The metastasis site was available in 100 cases, and the lesions were localized in the upper two third of the stomach in 58 (58%) cases, diffuse lesions in 28 (28%), whilst 14 (14%) patients had the main lesions confined in the antrum. The endoscopic description was available in 58 patients, and the metastasis presented as linitis plastica in 27 (47%), ulceration in 12 (21%) submucosal mass in 8 (14%), multiple erosions in 5 (9%), gastritis in 4 (7%), and as polyps in the remaining 2 patients. The main presenting symptom, extrapolated from 133 patients, was vomiting (43 cases; 32%), dyspeptic symptoms (42 cases; 31%), dysphagia (28 cases; 21%), anaemia/bleeding (19 cases; 15%), and perforation (1 case).
Discussion
It is known that the stomach represents an infrequent site of tumor metastases (30). In particular, metastases originating from primary gynaecological cancers are particularly rare, although those from the breast appear to be less uncommon (6). Indeed, the incidence of gastric metastases was 3.6% on 694 patients with breast cancer (7). In addition, the breast cancer was the most prevalent cause of gastric metastases (27%), followed by lung cancer (23%), renal cell cancer (7.6%) and malignant melanoma (7%) in a recent review (4). On the other hand, metastases from gastric cancer to either breast or uterus are exceptional (31,32), whilst, ovarian metastases secondary to gastric cancer—the so called Krukenberg tumor—has been extensively described in the literature (33). Synchronous or metachronous presence of primary gastric cancer and primary gynaecological tumor (ovary, uterus, and breast) has been described in 15 (0.6%) cases out of 2,668 patients with gastric cancer (34). Of note, it has been also found that patients with a personal history of uterine cancer are at 1.38-fold (95% CI: 1.09–1.72) increased risk of developing primary gastric cancer (35). Finally, women with hereditary inactivating mutations in the E-cadherin gene CDH1 are at increased risk for both gastric cancer and breast carcinoma (6). All these observations would indicate an intriguing link between gastric and gynaecologic organs carcinogenesis.
The present study described 3 new cases of gastric metastases and we reviewed data of literature on such a topic. Overall, we analyzed data 18 patients with gastric metastases from the ovary, 11 from the uterus, and 159 from breast cancer. Gastric metastases from uterine tumors appear to be less frequent. However, an autopsy series on 414 females with primary uterine tumors found a prevalence of 4.6%, a rate comparable with the 4.1% found on 101 patients with ovarian cancer (3). In the same study, the breast metastasis rate was found to be 11.6%.
Based on the detailed data we collected, a reliable comparison is possible only between ovarian and breast metastasis pattern. We found that the mean age at metastasis diagnosis was distinctly lower in breast than in ovarian cancer females (47 vs. 59 years), the mean interval for metastasis onset was longer (78 vs. 32 months), the gastric lesions were localized more frequently in the upper stomach (58% vs. 28%), and the most frequent endoscopic feature was linitis plastica (47%) for breast metastases and submucosal lesions for ovarian metastases (61%). Moreover, the most prevalent symptoms were vomiting (32%) in breast cancer metastasis and dyspeptic symptoms/asymptomatic in the ovarian cancer (61%). Overall, a synchronous gastric lesion was detected in only few cases, whilst in the majority of patients a metachronous metastasis developed, with an onset as long as 33 years in a breast cancer case (27). Of note, a metastatic breast cancer was diagnosed in patients with an endoscopic feature of “gastritis” (4 cases) or simple “erosions” (4 cases) (21), suggesting the need of adequate biopsy sampling in these patients. In our case, the histological diagnosis of breast cancer metastasis in the stomach was achieved only at third endoscopic sampling, despite the macroscopic suspect of neoplastic lesion. Such a finding is not uncommon, the failure of histological diagnosis on endoscopic biopsies being reported in 8–9% of cases (3,15). Finally, we found that the main lesions of gastric metastases are localized in either gastric body or fundus more frequently than in the antrum.
In conclusion, gastric metastasis mainly occurs from breast cancer. Both ovarian and uterine metastases are distinctly less frequent, but not impossible.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Menuck LS, Amberg JR. Metastatic disease involving the stomach. Am J Dig Dis 1975;20:903-13. [Crossref] [PubMed]
- Berge T, Lundberg S. Cancer in Malm? 1958-1969. An autopsy study. Acta Pathol Microbiol Scand Suppl 1977.1-235. [PubMed]
- Oda Kondo H. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy 2001;33:507-10. [Crossref] [PubMed]
- Namikawa T, Hanazaki K. Clinicopathological features and treatment outcomes of metastatic tumors in the stomach. Surg Today 2014;44:1392-9. [Crossref] [PubMed]
- Campoli PM, Ejima FH, Cardoso DM, et al. Metastatic cancer to the stomach. Gastric Cancer 2006;9:19-25. [Crossref] [PubMed]
- Bombonati A, Lerwill MF. Metastases to and from the Breast. Surg Pathol Clin 2012;5:719-47. [Crossref] [PubMed]
- Zhou JJ, Miao XY. Gastric metastasis from ovarian carcinoma: a case report and literature review. World J Gastroenterol 2012;18:6341-4. [Crossref] [PubMed]
- Kobayashi O, Sugiyama Y, Cho H, et al. Clinical and pathological study of gastric cancer with ovarian metastasis. Int J Clin Oncol 2003;8:67-71. [Crossref] [PubMed]
- Kim EY, Park CH, Jung ES, et al. Gastric metastasis from ovarian cancer presenting as a submucosal tumor: a case report. J Gastric Cancer 2014;14:138-41. [Crossref] [PubMed]
- Liu Q, Yu QQ, Wu H, et al. Isolated gastric recurrence from ovarian carcinoma: A case report. Oncol Lett 2015;9:1173-6. [PubMed]
- Hwangbo S, Kwon OK, Chung HY, et al. Improved survival of a patient with gastric and other multiple metastases from ovarian cancer by multimodal treatment: a case report. J Gastric Cancer 2015;15:218-21. [Crossref] [PubMed]
- Obeidat F, Mismar A, Shomaf M, et al. Gastric perforation secondary to metastasis from ovarian cancer: case report. Int J Surg Case Rep 2013;4:541-3. [Crossref] [PubMed]
- Moldovan B, Banu E, Pocreaţă D, et al. Gastric metastasis of cervix uteri carcinoma, rare cause of lower gastric stenosis. Chirurgia (Bucur) 2012;107:816-20. [PubMed]
- Kobayashi O, Murakami H, Yoshida T, et al. Clinical diagnosis of metastatic gastric tumors: clinicopathologic findings and prognosis of nine patients in a single cancer center. World J Surg 2004;28:548-51. [Crossref] [PubMed]
- De Palma GD, Masone S, Rega M, et al. Metastatic tumors to the stomach: clinical and endoscopic features. World J Gastroenterol 2006;12:7326-8. [Crossref] [PubMed]
- Jeladharan G, Subhalal N, Praseeda I. Gastric ulcer due to metastasis from carcinoma cervix. Indian J Gastroenterol 1992;11:88. [PubMed]
- Almubarak MM, Laé M, Cacheux W, et al. Gastric metastasis of breast cancer: a single centre retrospective study. Dig Liver Dis 2011;43:823-7. [Crossref] [PubMed]
- Tan L, Piao Y, Liu Z, et al. Breast cancer metastasis to the stomach confirmed using gastroscopy: A case report. Oncol Lett 2014;8:1205-7. [PubMed]
- Yagi Y, Sasaki S, Yoshikawa A, et al. Metastatic gastric carcinoma from breast cancer mimicking primary linitis plastica: A case report. Oncol Lett 2015;10:3483-7. [PubMed]
- Jones GE, Strauss DC, Forshaw MJ, et al. Breast cancer metastasis to the stomach may mimic primary gastric cancer: report of two cases and review of literature. World J Surg Oncol 2007;5:75. [Crossref] [PubMed]
- Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer 2000;89:2214-21. [Crossref] [PubMed]
- Yim H, Jin YM, Shim C, et al. Gastric metastasis of mammary signet ring cell carcinoma--a differential diagnosis with primary gastric signet ring cell carcinoma. J Korean Med Sci 1997;12:256-61. [Crossref] [PubMed]
- Winston CB, Hadar O, Teitcher JB, et al. Metastatic lobular carcinoma of the breast: patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol 2000;175:795-800. [Crossref] [PubMed]
- Nakamura J, Okuyama K, Sato H, et al. Repeated changes of the molecular subtype in gastric metastasis from breast cancer: A case report. Mol Clin Oncol 2016;4:695-8. [PubMed]
- Schwarz RE, Klimstra DS, Turnbull AD. Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol 1998;93:111-4. [Crossref] [PubMed]
- Tremblay F, Jamison B, Meterissian S. Breast cancer masquerading as a primary gastric carcinoma. J Gastrointest Surg 2002;6:614-6. [Crossref] [PubMed]
- Ayantunde AA, Agrawal A, Parsons SL, et al. Esophagogastric cancers secondary to a breast primary tumor do not require resection. World J Surg 2007;31:1597-601. [Crossref] [PubMed]
- Groisman GM, Depsames R, Ovadia B, et al. Metastatic carcinoma occurring in a gastric hyperplastic polyp mimicking primary gastric cancer: the first reported case. Case Rep Pathol 2014;2014:781318.
- Lorimier G, Binelli C, Burtin P, et al. Metastatic gastric cancer arising from breast carcinoma: endoscopic ultrasonographic aspects. Endoscopy 1998;30:800-4. [Crossref] [PubMed]
- Weigt J, Malfertheiner P. Metastatic Disease in the Stomach. Gastrointest Tumors 2015;2:61-4. [Crossref] [PubMed]
- He CL, Chen P, Xia BL, et al. Breast metastasis of gastric signet-ring cell carcinoma: a case report and literature review. World J Surg Oncol 2015;13:120. [Crossref] [PubMed]
- Kumar M, Kumar A, Maroules M, et al. Postmenopausal vaginal bleeding as initial presentation of gastric cancer: a case report with literature review of prognostic factors and treatment of krukenberg tumor. Ann Transl Med 2016;4:84. [PubMed]
- Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol 2006;30:277-99. [Crossref] [PubMed]
- Dinis-Ribeiro M, Lomba-Viana H, Silva R, et al. Associated primary tumors in patients with gastric cancer. J Clin Gastroenterol 2002;34:533-5. [Crossref] [PubMed]
- Teng CJ, Huon LK, Hu YW, et al. Secondary Primary Malignancy Risk in Patients With Cervical Cancer in Taiwan: A Nationwide Population-Based Study. Medicine (Baltimore) 2015;94:e1803. [Crossref] [PubMed]


