Clinical characteristics and molecular pathology of skull ectopic thyroid cancer
Introduction
Ectopic thyroid refers to a thyroid located in the front of trachea and outside fascia, and is a rare disease. Only one person is attacked by the disease in about every 100,000 to 300,000 people (1). The cause of the disease is that the thyroid migration during the embryonic period is disrupted, patients will become tongue thyroid or thyroglossal duct cyst with decline incompleteness, and the thyroid can also be migrated horizontally to the lateral neck or other region. Under normal conditions, the ectopic thyroid is the only thyroid gland existing in body. In exceptional circumstances, in addition to already existing ectopic thyroid, there may also be a second ectopic or orthotopic thyroid gland. It should be a high alert that the ectopic thyroid gland is prone to have pathological changes, which should be distinguished from orthotopic thyroid cancers. The paper reports two cases of ectopic thyroid cancers growing on the skull and molecular information pathway analysis was conducted by combing thyroid cancer-associated marker protein tissue microarray test results.
Clinical data
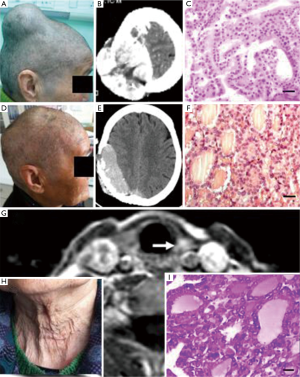
Example 1: a female patient was aged 74 years old, married, a farmer, and a Zhangjiagang citizen. She received a surgical treatment at the Neurosurgery Department of the Second Affiliated Hospital of Soochow University (No. 265135) from The Third People’s Hospital of Zhangjiagang City in March 2010 due to a tumor of 6 cm × 6 cm × 6 cm on head. In the surgery, the tumor was found located in a cap-like fascia and slightly adhered to the outer layer of cerebral dura mater, the inner layer was smooth, and the brain tissue was not invaded. Tumor central skull was completely absorbed and destroyed, the skull on the margin of the tumor presented a jagged shape and was eroded by tumor tissue. The whole block of the skull was cut out, and the skull was ground using an abrasive drilling into a normal skull, the meninges adhere to the tumor was cut off, and a titanium plate and an artificial meninges were used for reconstruction. The tumor volume was about 10 cm × 9 cm × 9 cm (500 g) and there was old blood clots in the center of the tumor which was pathologically diagnosed as thyroid cancer (follicular). Accordingly, the further neck examination MRI indicated that there was a strengthened signaling about 1 cm on the left lobe of the thyroid. A bilateral subtotal thyroidectomy was conducted and the pathological diagnosis showed nodular goiter. After the patient took “euthyrox”, the thyroid function was found normal and the patient did not receive other treatments. A follow-up was conducted to October 2014 (4 years and 7 months after surgery), the neck was normal, but another tumor was found in the head in situ. The patient underwent a second surgical resection in The Third People’s Hospital of Zhangjiagang City. The pathological diagnosis was the same with the first time and was also follicular thyroid cancer (Figure 1). The follow-up was continued until October 2015 and the patient was died of elderly multiple organ failure.
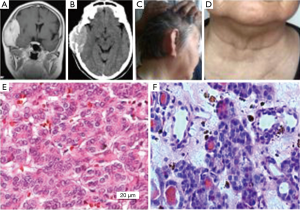
Example 2: a female patient was 65 years old, a Zhangjiagang citizen, and a farmer. The patient was hospitalized in the Neurosurgery of Zhangjiagang City Guanghe Hospital (No. 0138412) due to dizziness, vomiting, and right temporal tumor on March 9, 2015. The head MRI showed a tumor image in the right temporal region about 68 mm × 39 mm × 72 mm with a clear border. T1WI showed equal/low signal, T2WI showed equal/high signal, and FLAIR showed slightly high uniform signal, and small dot short T1 and short T2 signals were observed in the tumor. The lesion grew inside and outside the head from a midline, low signal was observed around the lesion, no obvious edema was observed, and the enhanced scanned lesions were evident, uniform and strengthened. After the admission, the patient was subjected to right temporal craniotomy under general anesthesia and the tumor was found under the cap-like fascia and outside the cerebral dura mater, and linked to the cerebral dura mater. The skull in the tumor region was severely damaged, the eroded skull and tumor are removed together, and the tumor was pathologically diagnosed as follicular thyroid cancer (No. 100263). The patient was subjected to thyroid tumor resection four years ago and the pathologic diagnosis was thyroid cell proliferation currently. The follow-up was conducted from December 2015 and the body examination showed that the head and neck conditions were normal (Figure 2).
Foreign case: according to the PubMed-MEDLINE database, the data of related cases of ectopic thyroid cancer were reviewed in Table 1.

Full table
Thyroid cancer-associated marker protein tissue microarray examination and result analysis
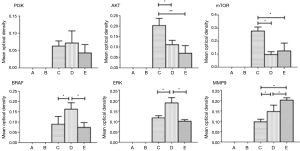
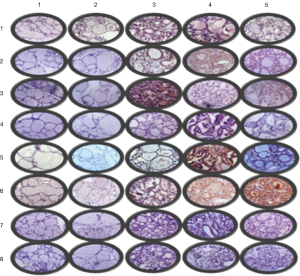
The tissue microarray was prepared according to the report by Xiao et al. (2). The scalp side, meninges side, tumor center, and tumor margins of the head tumor of the patient in example 1, as well as normal tissue at the junction, thyroid cancer, orthotopic thyroid cancer and thyroid metastatic cancer and other 10 tissues as controls were spotted on the holes of chip trays with three holes for each sample, and then the tissues were fixed. After the completion of slide preparation and slice cutting, the related protein markers in the signaling pathway of thyroid cancer were stained with EnVision immunohistochemical method: (I) PI3K (Santa Cruz Company, article number: SC-1637, dilution: 1:100); (II) Akt (Sigma company, article number: SAB4500799, dilution: 1:100); (III) mTOR (Santa Cruz company, article number: SC-8319, dilution: 1:100); (IV) BRAF (Sigma company, article number: HPA001328, dilution: 1:100); (V) ERK (Santa Cruz company, article number: SC-292838, dilution: 1:100); (VI) MMP-9 (Santa Cruz company, article number: SC-6840, dilution: 1:100); (VII) IKK (Abcam, article number: ab32041, dilution: 1:50); and (VIII) NFkB (Abcam, article number: ab137692, dilution: 1:500). The observed results of the immune complexes in an ordinary light microscope were showed in Figure 3 and analyzed using a specialized image analysis software (IPP6.0) (Figure 4). The obtained results showed that: (I) AKT (P=0.012, 0.002) and mTOR (P=0.002, 0.004) were highly expressed in the ectopic thyroid cancer; (II) BRAF (P=0.029, 0.014) and ERK (P=0.002, 0.001) were highly expressed in orthotopic thyroid cancer; (III) MMP-9 (P=0.023, 0.016) was highly expressed in metastatic thyroid cancer.
Three thyroid cancer regulatory molecule prediction
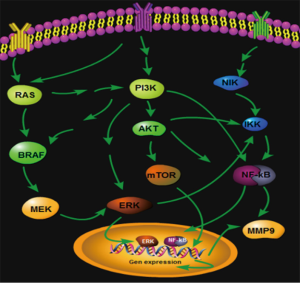
Many studies showed that the PI3K/Akt/mTOR signaling pathway was excessively activated in the incidence and the development of thyroid cancers. PI3K was an upstream key signal protein of rapamycin target proteins and a heterodimer consisting of catalytic subunit (Pl10) and regulatory subunit (P85) in cytoplasm (3,4). Among PI3K, the function of type I PI3K activated by the cell surface receptor was the most important. Accordingly, the PI3K gene was used as a key gene to be entered into the PATHWAY platform of data in the KEGG. By entering the PI3K gene, a number of signaling pathways associated with PI3K can be found. BRAF, NF-κB, PI3K, etc. were selected to compare with the genes in the molecular pathways of thyroid cancer reported in the literature and AKT, IKK, MMP9, and nearly 20 genes regulating the three thyroid cancers were regarded as target genes. The expression degree of each sample on the tissue microarray was verified by immunohistochemical staining, and was analyzed by using the IPPS 6.0 image analysis software and subjected to data processing, so that the conclusions in the abstract of this paper were obtained. Build Pathways software was used to draw the regulations in the Figure 5.
Discussion
Epidemiological data
The incidence of the thyroid cancers increases gradually with about 163,000 new cases around the world each year (5), and respectively accounts for the fifth and fourth place in the female malignant tumors in United States and China (Shanghai) (6). Such incidence refers to the disease occurring in the orthotopic thyroid and there were rare reports on the ectopic thyroid cancer. According to the nearly 30 years of Chinese literature summed up by Wang et al. (7) in 2012, there were a total of 198 cases of ectopic thyroid glands with lesions; and there were 3 cases of cervical ectopic thyroid cancers reported by Zhou et al. in 2014 (8); and there were a total of 203 cases wherein there were 43 cases of thyroid tumors (20%) and 36 cases of thyroid cancers (17%). In the foreign countries, we used five keywords of “thyroid gland”, “ectopic thyroid”, “thyroid cancer”, “ectopic thyroid cancer” and “metastatic thyroid cancer” to review a total of 109 relevant literature from 50 years (1964–2014) in the PubMed-MEDLINE database, including 39 literature of ectopic thyroid lesions and a total of 46 cases (9-13) (Table 1). From the Table 1, the ectopic thyroid gland is prone to cause disease and the glandular cancer incidence is up to 47% (21/46). The incidence of the glandular cancer and other benign lesions is followed by 17.4% (8/46) and patients with no lesions are found with only 37% (17/46). Although the incidence of orthotopic thyroid cancer shows a rapidly increasing trend (in Shanghai: 1.02/100,000 in 1960, 2.39/100,000 in 1972 and 3.80/100,000 in 1978), whether the incidence of ectopic thyroid cancer also increases is not known due to the lack of information. As for the ectopic thyroid cancer growing on skull, the incidence is rarer. In China, Chen (14) and Xu et al. (15) respectively reported one case in 1994 and 2004. No reports on the skull ectopic thyroid cancer were found in the foreign countries. Although Vrachimis et al. (16) reported one case diagnosed with thyroid cancer by surgical resection of brain and thyroid nodules by pathology, the patient was also found with multiple lesions in body bone, lung and soft tissue imaging at the same time. The desired effect of the disappearance of all the lesions after treatment was found, so that the case did not belong to the skull ectopic thyroid cancer, but a thyroid cancer with multiple systemic metastasis. In view of this, the differential diagnosis of the ectopic thyroid cancer and the metastatic thyroid cancer is worthy of discussion.
Diagnosis and treatment of ectopic thyroid cancer
The prerequisite of the diagnosis of ectopic thyroid cancer is a normal or absent orthotopic thyroid, or the orthotopic thyroid gland presents a benign lesion, not a cancer. Ectopic thyroid cancer can only be diagnosed if the ectopic thyroid gland appears canceration; if the ectopic thyroid gland appears benign tumor, then it should be diagnosed as ectopic thyroid tumor; and if the tissue is normal thyroid tissue, then it should be diagnosed as ectopic thyroid gland. The metastatic thyroid cancer needs to be identified is an orthotopic thyroid gland and the canceration occurs before the metastasis occurs. If occult lesion on the thyroid gland in situ, it is possible that firstly the metastatic thyroid cancer lesions would be mistaken for ectopic thyroid cancer. At this time, it is of absolutely necessary to conduct a comprehensive inspection of the orthotopic thyroid gland. According to this standard, the orthotopic thyroid glands of 21 patients with area outside of the orthotopic thyroid gland occurring thyroid cancer were analysed. The results showed there were 16 cases of patients with orthotopic normal thyroid (yes), two cases of patients with orthotopic abnormal thyroid (no), one case of orthotopic thyroid adenoma, and the remaining two cases with orthotopic thyroid gland with unknown condition in this paper. The two female elderly patients reported herein had orthotopic thyroid gland all occurring benign adenomatous changes. The ectopic thyroid glands were all use the skull as a center and expanded to the edges to form a huge extra-cerebral space-occupying lesion. The skull had erosion destroy, but no invasion was found under cerebral dura mater and outside cap-like fascia. After the orthotopic and ectopic thyroid lesions were removed, no thyroid function decline was caused. The patients were subjected to conventional oral levothyroxine sodium and did not receive chemotherapy. After the follow-up conducted for 4 years, the patient in the example 1 was subjected to surgical resection again due to local recurrence of the ectopic thyroid cancer, the in situ thyroid adenoma was not recurrent, but the patient died of age-related multiple organ failure in 5 years and 8 months after surgery. The patient in the example 2 found no evidence of recurrence orthotopic and ectopic thyroid glands.
Thyroid cancer molecular signal pathways
Goyal’s (17) report showed that the incidence and invasion of orthotopic follicular thyroid carcinoma were closely related with high expression of serine-threonine kinase BRAF activating MAPK pathways. But the ectopic thyroid cancer, particularly the ectopic thyroid cancer growing on the skull, is a rare disease, and no reports on related molecular pathways have yet to be found (18). According to the information screen on the self-made tissue microarray and single-specimen paraffin slice verification, and by combining IPPS 6.0 image analysis software (19) and statistical processing (Figures 3 and 4), the results showed that the Akt and mTOR protein expressions in the Akt-mTOR signaling pathway of skull growing ectopic thyroid cancer were significantly higher than those in the orthotopic and metastatic thyroid cancer (P=0.012, 0.002) and (P=0.002, 0.004). The Akt and mTOR expressions were slightly lower in the orthotopic and metastatic thyroid cancers without significant differences (P=0.162, 0.433). In the orthotopic thyroid cancer, BRAF and ERK of the MAPK signaling pathway were highly expressed and the expression rates were obviously higher than those in the ectopic and metastatic thyroid cancers (P=0.029, 0.014; P=0.002, 0.001). However, no significant differences in the BRAF and ERK were found in ectopic and metastatic thyroid cancers (P=0.573, 0.271). MMP-9 had a highest expression in the metastatic thyroid cancer, a moderate expression in the orthotopic thyroid cancer, and a lowest expression in ectopic thyroid cancer (P=0.023, 0.016).
Taken together, there were multiple activated signaling pathways in orthotopic thyroid cancer, ectopic thyroid cancer, and metastatic thyroid cancer, but the expressions were significantly different, indicating that the three thyroid cancer may be regulated by different signaling pathways (Figure 5). PI3K was a crossing gene in the three signaling pathways and showed no significant difference (P=0.692, 0.388, 0.227). However, the PI3K had regulation functions for Akt/mTOR, MAPK, and NF-κB signaling pathways. According to the information, we speculated that the PI3K is an important starting gene in thyroid cancer. After the canceration starts, due to the fact that the local microenvironments of thyroid cancers in different parts are different, the thyroid cancers are regulated by different signaling pathways. The ectopic thyroid cancer was correlated with Akt/mTOR pathway activation; orthotopic thyroid was related with MAPK/BRAF/ERK signaling pathway activation; and the metastatic thyroid cancer was related with NF-κB/MMP9 activation. The three different signal pathways control the development of the thyroid cancers with different properties, which is of a certain reference value for the further study of thyroid cancer targeted molecule treatment.
Acknowledgements
Funding: This work was supported by Scientific and Technological Development Program of Suzhou, China (SYS201477); University Natural Science Foundation of Jiangsu, China (15KJB320011).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Cordes S, Nelson JJ. Papillary carcinoma arising in median ectopic thyroid tissue: management of the thyroid gland. Ear Nose Throat J 2010;89:E4-7. [PubMed]
- Xiao H, Huang Q, Dong J, et al. The analysis on the relationship between expressions of ABCG2, VEGF, IGFBP-2 and EGFR in glioma tissue microarray and prognosis. Chinese Journal of Neuromedicine 2007;6:878-82.
- Wang SC, Chai DS, Chen CB, et al. HPIP promotes thyroid cancer cell growth, migration and EMT through activating PI3K/AKT signaling pathway. Biomed Pharmacother 2015;75:33-9. [Crossref] [PubMed]
- Xu J, Cai J, Jin X, et al. PIG3 plays an oncogenic role in papillary thyroid cancer by activating the PI3K/AKT/PTEN pathway. Oncol Rep 2015;34:1424-30. [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Nix P, Nicolaides A, Coatesworth AP. Thyroid cancer review 2: management of differentiated thyroid cancers. Int J Clin Pract 2005;59:1459-63. [Crossref] [PubMed]
- Wang H, Sun Y, Jia J. Analyzing for 198 cases of ectopic thyroid glands. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2012;26:814-6. [PubMed]
- Zhou Y, Qian M, Zhou Z, et al. Diagnosis and treatment of primary ectopic thyroid carcinoma: report of 3 cases and literature review. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2014;28:151-3. [PubMed]
- Pagano L, Caputo M, Samà MT, et al. Unusual metastases from tall cell variant of papillary thyroid cancer. Head Neck. 2013;35:E381-5. [Crossref] [PubMed]
- Wu ZX, Zheng LW, Dong YJ, et al. Modified approach for lingual thyroid transposition: report of two cases. Thyroid 2008;18:465-8. [Crossref] [PubMed]
- Spears RP, Wei JP. Nonmalignant ectopic thyroid tissue. Am Surg 1993;59:133-5. [PubMed]
- Misaki T, Koh T, Shimbo S, et al. Dual-site thyroid ectopy in a mother and son. Thyroid 1992;2:325-7. [Crossref] [PubMed]
- Okstad S, Mair IW, Sundsfjord JA, et al. Ectopic thyroid tissue in the head and neck. J Otolaryngol 1986;15:52-5. [PubMed]
- Chen CZ. A case of ectopic thyroid carcinoma in the left parietal occipital region. Chin J Neurosurg 2004;20:431.
- Xu HB, Feng GS, Qiu GZ, et al. Intracranial ectopic thyroid with partial malignant transformation: a case. Journal of Practical Radiology 1994;10:566.
- Vrachimis A, Schmid KW, Jürgens H, et al. Cerebral metastases from thyroid carcinoma: complete remission following radioiodine treatment. Deutsches Ärzteblatt International 2013;110:861-6. [PubMed]
- Goyal N, Setabutr D, Abdulghani J, et al. Molecular and genetic markers of follicular-cell thyroid cancer: etiology and diagnostic and therapeutic opportunities. Adv Exp Med Biol 2013;779:309-26. [Crossref] [PubMed]
- Giunti S, Antonelli A, Amorosi A, et al. Cellular signaling pathway alterations and potential targeted therapies for medullary thyroid carcinoma. Int J Endocrinol 2013.803171. [PubMed]
- Kraan MC, Smith MD, Weedon H, et al. Measurement of cytokine and adhesion molecule expression in synovial tissue by digital image analysis. Ann Rheum Dis 2001;60:296-8. [Crossref] [PubMed]