Clinical significance of cell population data (CPD) on Sysmex XN-9000 in septic patients with our without liver impairment
Introduction
Infections are leading causes of morbidity and mortality around the globe, especially in low income countries. According to recent statistics, the burden of infectious diseases is still so high that as many as 10 million persons die each year for these conditions (1). Despite these sizeable figures, the biomedical research for identifying reliable biomarkers to help the diagnosis, prognostication and therapeutic monitoring of infectious diseases is still much lower than that for other less prevalent conditions such as cancer, diabetes and cerebrovascular disorders (1). Sepsis (also known as septicemia) is indeed the most severe complication in patients with infections. Sepsis is commonly defined by the United States (US) Centers for Disease Control and Prevention (CDC) as a generalized, overwhelming and life-threatening response to infection, which can lead to tissue damage, organ failure, up to death (2). Sepsis is typically caused by an immune response triggered by different types of pathogens, including bacteria, fungi, viruses and even parasites (3). Upon information collected for billing purposes, the CDC has recently estimated that the number of patients hospitalized for sepsis in the US has increased from 621,000 in the year 2000 up to 1,141,000 in 2008 (2), thus confirming the high prevalence of this condition even in high income countries. Even more importantly, the mortality for sepsis remains considerably high, with nearly 30% of patients dying for septicemia in the intensive care unit (ICU) (4).
Although an early diagnosis of sepsis is pivotal for improving patient outcome, essentially by adoption of appropriate therapeutic (e.g., antibiotic, antiviral or antimycotic) and supportive measures, the diagnostic approach and monitoring of patients with sepsis remains challenging, even using the criteria defined by the International Sepsis Definitions Conference (ISDC) (5). More specifically, a number of biomarkers have been proposed for the timely diagnosis and prognostication of septic patients, including lactic acid, procalcitonin (PCT), C-reactive protein (CRP), immature granulocytes (IG) and the delta neutrophil index (DNI) (6-9). Interestingly, most of these parameters present a number of drawbacks for routine assessment in septic patients, such as insufficient diagnostic performance and considerable costs (especially PCT), whereas the clinical information still published so far for others (e.g., IG and DNI) does not allow a widespread introduction in clinical practice, thus paving the way for additional research on this topic.
The new generation of hematological analyzer has taken advantage from a number of technological innovations, which have allowed to expand the panel of potential information available from the complete blood count (CBC). In particular, novel parameters of the leukocyte count and differential are emerging as potentially useful markers in a number of human disorders. These basically include IG (10,11), or the high fluorescence lymphocyte cells (HFLCs) count, a parameter which is usually related to the presence of activated lymphocytes, plasma cells and other blast cells (12). Additional research parameters characterizing different leukocyte populations [i.e., cell population data (CPD)] such as neutrophils (NE), lymphocytes (LY) and monocytes (MO) have recently became available, and preliminary observations suggest that their assessment may be useful in the diagnosis of myelodysplastic syndromes (13,14) and sepsis (15).
The new Sysmex XN-9000 analyzer is capable to provide an extended leukocyte differential count with as many as 22 CPD parameters that can be generated along with to the CBC. Therefore, the aim of this study was to evaluate the clinical significance of novel CPD parameters as putative biomarkers for early diagnosis of septic patients and their follow-up in the ICU. Special focus has also been placed on liver function, since patients with hepatic failure frequently have an impaired inflammatory response and are more vulnerable to worse outcomes.
Methods
Subject population
The study population included 115 adult patients admitted to the ICU of the general hospital of Bergamo, Italy (Papa Giovanni XXIII Hospital), between February and March 2014. A single inclusion criterion was used (i.e., age ≥18 years), although all patients with a positive history of hematologic disorders and/or hospitalization in the previous 48 hours were excluded from the study. Clinical data, including signs and sites of concomitant infection (recognized or suspected) and presence/absence of liver impairment classified according to Model for End-Stage Liver Disease (MELD) score (16) were recorded upon ICU admission.
In the day of ICU admission, patients underwent routine laboratory testing, entailing a large panel of blood tests as well as two daily clinical evaluations until ICU discharge. The information collected with clinical assessment included the presence/absence of sepsis using the ISDC guidelines (5), as well as the degree of severity by calculation of the Sequential Organ Failure Assessment (SOFA) score (17). According to the presence/absence of both sepsis and liver dysfunction, the patients were hence classified as follows: no sepsis (NS), presence of sepsis (SE) and presence of septic shock (SS). These three groups of patients were further sub-classified as having or not liver dysfunction.
The study also included 200 ostensibly healthy blood donors [healthy subjects (HS); 100 men and 100 women] aged between 18–70 years (average: 43.0 years; 95% CI, 41.0–45.0 years), who underwent routine blood testing before a regular blood donation during the same period.
Sample preparation and methods
Blood samples were collected in K3EDTA evacuated plastic tubes (Becton Dickinson, Franklin Lakes, NJ, USA) and were then analyzed using XN-9000 (Sysmex Co., Kobe, Japan), within 2 hours from sample collection. The main parameters evaluated in this study included the CPD of NE, LY and MO leukocyte parameters populations, which provide information related to cell complexity, fluorescence intensity, cell size and width of dispersion of the events measured on the three axis of WDF channel. A brief explanation on the morphological and functional significance of these CPD parameters is summarized in Table 1. The following parameters are reported on the x-axis: neutrophils cell complexity (NE-SSC), lymphocytes cell complexity (LY-X), monocytes cells complexity (MO-X), neutrophils complexity and width of dispersion of the events measured (NE-WX), lymphocytes complexity and width of dispersion of the events measured (LY-WX) and monocytes complexity and width of dispersion of the events measured (MO-WX) (see Table 1); the following parameters are reported on the y-axis: neutrophils fluorescence intensity (NE-SFL), lymphocytes fluorescence intensity (LY-Y), monocytes fluorescence intensity (MO-Y), neutrophils fluorescence intensity and the width of dispersion (NE-WY), lymphocytes fluorescence intensity and the width of dispersion (LY-WY) and MO-WY (see Table 1); whereas the following parameters are reported on the z-axis: neutrophils cell size (NE-FSC), lymphocytes cell size (LY-Z), monocytes cell size (MO-Z), neutrophils cell size and the width of dispersion (NE-WZ), lymphocytes cell size and the width of dispersion (LY-WZ) and monocytes cell size and the width of dispersion (MO-WZ) (see Table 1).
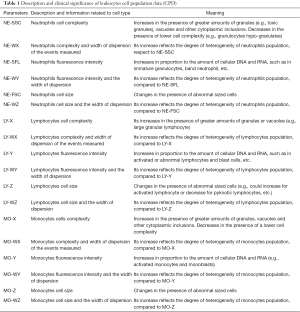
Full table
The within-run imprecision of CPD on XN-9000 was also preliminary assessed using ten replicates of five fresh whole blood routine samples, displaying a coefficient of variation (CV) always lower than 10% for all parameters.
Statistical analysis
For each patient were considered for CPD data analysis only single blood samples. For NS the second day of patients admission in ICU, for SE and SS the first day of sepsis/septic shock condition of the patient.
The distribution of values of the three different classes of patients (i.e., NS, SE, SS) was assessed with Shapiro-Wilk test. The preliminary analysis of data revealed a non-normal distribution of values, so that results were reported as median for each class of subjects. The statistical difference was then evaluated with the nonparametric Kruskal-Wallis and Steel-Dwass-Critchlow-Fligner (pair comparison) tests.
The diagnostic accuracy of the CPD parameters was evaluated against the criteria defined by the ISDC (5) by means of receiver operating characteristics (ROC) curves, in which data of SE and SS patients were compared with those of the NS population.
The correlation between the CPD parameters and SOFA score was evaluated on data collected through all time period of admission and evaluated by Spearman correlation, a P<0.05 was considered positive. The statistical analysis was performed with Analyse-it (Analyse-it Software Ltd., Leeds, UK). This study was carried out in accordance with the Declaration of Helsinki, and under the terms of all relevant local legislation. The investigation was based on pre-existing samples, so that ethical permission and informed consent were unnecessary.
Results
Population characteristics
Thirty one (27%) out of 115 patients originally included in the study ought to be excluded for the presence of hematologic diseases (15 patients), or because of the overall period of ICU stay was lower than 48 hours (16 patients), so impeding complete collection of data. The final study population thus included 84 patients, 44 (52%) of whom did not develop sepsis, whereas the remaining 40 (48%) developed sepsis (n=24) or SS (n=16). According to the MELD score, 18 (21.4%) ICU patients were classified as having liver impairment. No significant difference of gender, age and body mass index were observed in septic patients with or without liver impairment (data not shown), whereas the SOFA score was significantly higher in patients with liver dysfunction (11.0; 95% CI, 9.0–12.0) than in those without (4.0; 95% CI, 4.0–4.0; P<0.001).
CPD parameters for diagnosing sepsis in patients with or without liver dysfunction
With the exception of NE-FCS and LY-Z, the values of all CPD were found to be significantly different in ICU patients compared to HS (Table 2).
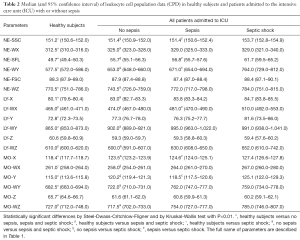
Full table
The values of NE-SFL and MO-X were also significantly higher in the SS group compared to SE and NS (Table 2). Interestingly, the values of many parameters were also found to be different in patients with or without liver impairment (Table 3). This was especially evident for the values of MO-Y and monocytes fluorescence intensity and the width of dispersion (MO-WY), whereas the CPD of the LY population (especially LY-X, LY-WX, LY-Z and LY-WZ) did not display a clear trend (Table 3). The trend of LY-Y was paradigmatic when separately analyzed in patients with or without liver impairment. In patients without liver impairment, the value in the SS group was considerably lower than in the NS and SE groups. Conversely, in patients with liver impairment the value was considerably higher in the SS group compared to the NS and SE groups.

Full table
As regards the diagnostic performance of CPD parameters, the ROC curves for diagnosing sepsis in ICU patients were characterized by a rather heterogeneous efficiency (Table 4). Overall, MO-X and NE-SFL exhibited the best diagnostic performance in all ICU patients as well as in those with or without liver impairment, whereas the AUCs of all the other parameters were definitely lower and often not reaching statistical significance.
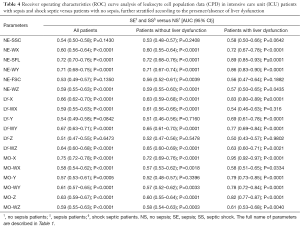
Full table
CPD parameters for predicting severity of sepsis in patients with or without liver dysfunction
The SOFA score assessment enabled the classification of ICU patients in four main classes (i.e., <5, from 6 to 10, from 11 to 15 and >15). The values of the various CPD parameters were significantly different across the different SOFA scores (data not show), interestingly in patients admitted in ICU, NE-SFL and MO-X parameters showed a positive correlation with the SOFA score see Table 5. The correlation improve in patient with liver impairment (i.e., rs =0.30 for NE-SFL and rs =0.35 for MO-X with P<0.0001) and in sepsis and SS patients (rs =0.35 for NE-SFL and 0.40 for MO-X with P<0.0001) (Table 5).
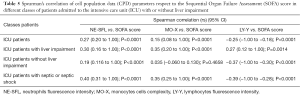
Full table
The LY-Y parameter showed a particular trend with negative correlation respect to SOFA score in patients without liver impairment (rs =−0.37; P<0.0001) conversely in patients with liver impairment the correlation was positive (rs =0.27; P<0.0001).
Conclusions
Despite remarkable efforts made over the past decade to identify reliable biomarkers that may allow a timely and accurate diagnosis of sepsis, the identification and prognostication of this condition still challenges the mind of many clinicians (3). As for biomarkers of other human disorders (18), the various biochemical parameters that have been proposed so far are characterized by a number of limitations, including an insufficient diagnostic performance, high cost and even a longer turnaround time that would make them unsuitable for urgent management of patients in the ICU. Therefore, the use of innovative, easy and virtually inexpensive parameters represents an appealing perspective for improving the clinical outcome in septic patients.
The results of our study first show that patients admitted to the ICU without a diagnosis of sepsis display rather different values of all CPD parameters compared to a healthy control population. A notable exception was represented by NE-FSC and LY-Z, since the value distribution of these parameters exhibits a large dispersion a consistent overlap with healthy subjects (Tables 2,3). Another interesting finding emerged from our study, is that the presence or absence of liver impairment in ICU patients has a considerable influence on the vast majority of CPD parameters. Nevertheless, an interesting trend was observed for the parameters NE-SFL and MO-X, wherein their values were found to be gradually increased by comparing NS patients with both the SE and SS populations (Tables 2,3). Interestingly, although these findings are substantially in accord with data previously published by Park et al. (15), the diagnostic performance obtained in our study appeared to be much better than those earlier shown (Tables 2,3,4). Indeed, the main difference with the previous study is attributable to the fact that we used a different approach for ROC curve analysis, wherein the reference population for estimating the diagnostic performance of CPD in our investigation was represented by a population of ICU patients without sepsis rather than a healthy population. According to a genuine clinical perspective, this strategy seems hence to more closely mirror the everyday challenge of diagnosing sepsis in the ICU.
At variance with previous studies, we also evaluated the clinical significance of CPD parameters in ICU patients with or without liver dysfunction. Interestingly, both LY and MO data displayed peculiar trends in ICU patients without or with liver impairment (Tables 2,3,4), exhibiting diagnostic performances that were rather different in each of these two populations (Table 4). Such trend could be explained by the different inflammatory response in hepatopathic septic patients, as currently reported in the scientific literature (19). Taken together, this data highlights the need of additional research aimed to verify the diagnostic performance of all quantitative and qualitative leukocyte parameters in septic patients according to the presence or absence of liver dysfunction, wherein this condition may have a remarkable influence on leukocyte biology (e.g., by impairing production, survivor or both) (20).
An additional important information emerging from this study is that the convincing association between some CPD parameters (i.e., NE-SFL and MO-X) and the SOFA score discloses a potential and interesting application for prognostication of septic patients in the ICU, since these indices would apparently reflect a clinical worsening. Interestingly, NE-SFL displayed similar trends in both patients with or without liver dysfunction, so that they may be potentially used interchangeably for predicting clinical worsening. On the other hand, data emerged with measurement of LY-Y in the same patient population suggest that this parameter should only be used when a sub-classification according to the liver function is available (Table 5). This paradigmatic trend may also be explained by the different immune response that has been convincingly demonstrated in patients with or without liver impairment (21,22).
The results of this study suggest that some novel CPD parameters generated by Sysmex XN-9000 (namely NE-SFL and MO-X) may provide clinically useful information for diagnosis and management of sepsis in the ICU. Importantly, their diagnostic performance seems to be at least in part related to the liver function of such patients, so that their use should always take into consideration whether liver dysfunction is present or not. It is also noteworthy that CPD parameters are automatically generated along with the CBC on Sysmex XN-9000, so that their measurement would enable to obtain rapid and virtually inexpensive information to the clinicians.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The investigation was based on pre-existing samples, so that ethical permission and informed consent were unnecessary.
References
- Lippi G, Plebani M. Biomarker research and leading causes of death worldwide: a rather feeble relationship. Clin Chem Lab Med 2013;51:1691-3. [Crossref] [PubMed]
- Hall MJ, Williams SN, DeFrances CJ, et al. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief 2011.1-8. [PubMed]
- Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity 2014;40:463-75. [Crossref] [PubMed]
- Nguyen HB, Rivers EP, Abrahamian FM, et al. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med 2006;48:28-54. [Crossref] [PubMed]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530-8. [Crossref] [PubMed]
- Reinhart K, Bauer M, Riedemann NC, et al. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev 2012;25:609-34. [Crossref] [PubMed]
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55. [Crossref] [PubMed]
- Park BH, Kang YA, Park MS, et al. Delta neutrophil index as an early marker of disease severity in critically ill patients with sepsis. BMC Infect Dis 2011;11:299. [Crossref] [PubMed]
- Seok Y, Choi JR, Kim J, et al. Delta neutrophil index: a promising diagnostic and prognostic marker for sepsis. Shock 2012;37:242-6. [Crossref] [PubMed]
- Maenhout TM, Marcelis L. Immature granulocyte count in peripheral blood by the Sysmex haematology XN series compared to microscopic differentiation. J Clin Pathol 2014;67:648-50. [Crossref] [PubMed]
- Briggs C, Longair I, Kumar P, et al. Performance evaluation of the Sysmex haematology XN modular system. J Clin Pathol 2012;65:1024-30. [Crossref] [PubMed]
- Arneth BM, Ragaller M, Hommel K, et al. Novel parameters of extended complete blood cell count under fluorescence flow cytometry in patients with sepsis. J Clin Lab Anal 2014;28:130-5. [Crossref] [PubMed]
- Le Roux G, Vlad A, Eclache V, et al. Routine diagnostic procedures of myelodysplastic syndromes: value of a structural blood cell parameter (NEUT-X) determined by the Sysmex XE-2100™. Int J Lab Hematol 2010;32:e237-43. [Crossref] [PubMed]
- Furundarena JR, Araiz M, Uranga M, et al. The utility of the Sysmex XE-2100 analyzer's NEUT-X and NEUT-Y parameters for detecting neutrophil dysplasia in myelodysplastic syndromes. Int J Lab Hematol 2010;32:360-6. [Crossref] [PubMed]
- Park SH, Park CJ, Lee BR, et al. Sepsis affects most routine and cell population data (CPD) obtained using the Sysmex XN-2000 blood cell analyzer: neutrophil-related CPD NE-SFL and NE-WY provide useful information for detecting sepsis. Int J Lab Hematol 2015;37:190-8. [Crossref] [PubMed]
- Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464-70. [Crossref] [PubMed]
- Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care 2008;12:R161. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C. The biomarker paradigm: between diagnostic efficiency and clinical efficacy. Pol Arch Med Wewn 2015;125:282-8. [PubMed]
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:2063. [Crossref] [PubMed]
- Qamar AA, Grace ND. Abnormal hematological indices in cirrhosis. Can J Gastroenterol 2009;23:441-5. [Crossref] [PubMed]
- Chen X, Ye J, Ye J. Analysis of peripheral blood lymphocyte subsets and prognosis in patients with septic shock. Microbiol Immunol 2011;55:736-42. [Crossref] [PubMed]
- Alves-Filho JC, de Freitas A, Spiller F, et al. The role of neutrophils in severe sepsis. Shock 2008;30 Suppl 1:3-9. [Crossref] [PubMed]


