Tracheoesophageal fistula induced by invasive pulmonary aspergillosis
Introduction
Invasive pulmonary aspergillosis (IPA) often develops in severely immunocompromised patients which is associated with a high rate of short-term mortality. The classic risk factor for IPA is neutropenia, and the likelihood of IPA correlates with its duration and depth. Although exposure to aspergillus conidia through inhalation is common, tracheoesophageal fistula (TEF) induced by IPA has seldom been reported yet. It is very hard to treat the patient with IPA and TEF. A multidisciplinary care may improve the outcomes.
Case presentation
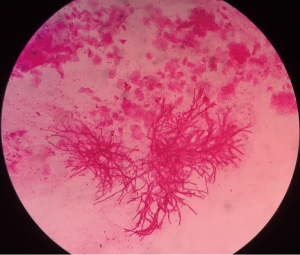
A 54-year-old male who had been diagnosed with aplastic anemia for 5 years presented to our intensive care unit (ICU) with dyspnea for two weeks. He also complained chest discomfort and intermittent hemoptysis for about one month. On physical examination, he appeared alert, cyanotic with fever up to 38.4 °C and inspiratory wheezing could be clearly heard in his main airway but respiratory sounds were diminished on the right upper lobe. Laboratory findings on admission were as follows: leukocyte 0.15×109/L, absolute neutrophil count 0.01×109/L, hemoglobin 47 g/L, platelet 2×109/L, galactomannan 1.45 µg/L, β-D-glucan 159.6 pg/mL. Chest computed tomography (CT) performed at admission showed airway constriction and halo sign in right upper lobe (Figure 1). Aspergillus hyphae could be detected in sputum direct smear largely that revealed the patient was suffering IPA (Figure 2). Because of the low level of platelet and high risk of bleeding, the patient refused bronchofiberscopy. Voriconazole which was the antifungal agent of choice for treatment of IPA according to almost all guidelines based on a significant mortality benefit was initiated (intravenous medication, 200 mg q12 h) (1). The symptoms of chest discomfort and hemoptysis persisted despite high-flow nasal cannula oxygen therapy for one week.
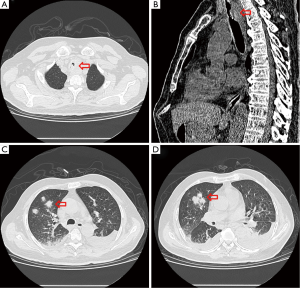
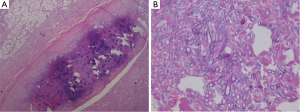
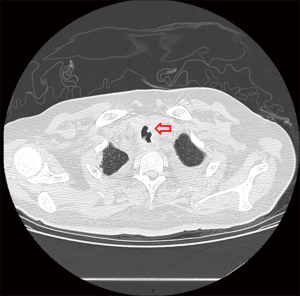
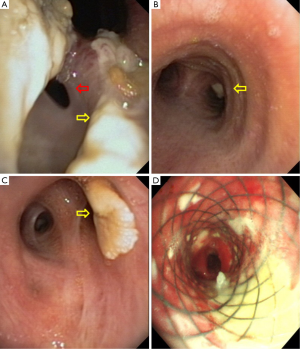
One week after ICU admission, a yellow-red lump measuring 3 cm in diameter was coughed up. Hemoptysis was heavily and relieved after hemocoagulase was given for 3 days. The patient’s abdomen was distended with the high-flow nasal cannula oxygen therapy. Histopathological examination of the lump presented necrosis tissue of the airway wall with typical aspergillosis hyphae (Figure 3). Another chest CT scan was ordered the next day which reported TEF (Figure 4). Aspergillus might be responsible for the result. Bronchoscopy detected the fistula (approximate 1.5 cm diameter) and extensive pseudomembrane formation around tracheal membrane. Histopathological examination of the pseudomembrane also revealed typical aspergillosis hyphae. Right principal bronchi was occluded >50% by polypoid granulation as well as right superior lobar bronchus (Figure 5). Bronchoalveolar lavage fluid (BALF) galactomannan was also tested and the result was for beyond the upper normal limit (5 µg/L).
Because of the poor performance condition and short life expectancy without proper treatment, multidisciplinary team working was provided. The panel consists of intensivists, thoracic surgeons, infectious disease specialists, respiratory specialists, microbiologists and clinical pharmacists. First, antifungal treatment was strengthened, amphotericin B (5 mg/kg) combined with voriconazole was also prescribed. Second, piperacillin/tazobactam was also prescribed (4.5 g q12 h) to prevent aspiration pneumonia duo to severe reflux. General treatment, such as indwelling jejunal-tube and intravenous hyperalimentation were also ordered.
In these patients, we decided to insert an airway stent alone, the main reasons are as follows: (I) the TEF sat in the upper esophageal which was proximal to esophageal entrance; (II) pseudomembranes were found in trachea by bronchoscopy which led to airway stenosis. Esophageal stent alone could not solve the symptom; (III) the persistent pressure to the wall of esophagus and airway after esophageal stenting is also an important cause which may leads to TEF; a straight and hourglass-shaped stents was placed by thoracic surgeons (Figure 5D), which was the most important thing to the patient. The stent was not removed due to the large size of fistula which was hard to heal and the stent was in good position verified by bronchoscopy in June this year. Complications of stenting, such as stent migration, granulation tissue formation, and retained secretions were not identified during follow up for one year.
Discussion
Acquired TEF may be induced by infection with different microorganisms such as tuberculosis, actinomycosis or malignant tumors (2). TEF caused by IPA is rare and seldom be reported. The only case reported by Joseph in 1994 was a child with bone marrow transplantation who had a poor outcome without intervention therapy (3). IPA is associated with a high rate of short-term mortality. The one-year mortality in IPA patients was up to higher than 70% and the cause of death in IPA patients not only included anoxia and respiratory failure, but also tracheal bronchus impairing (4). Despite marked improvements in bronchoscopic interventional treatment as well as antifungal treatment, the majority of patients with TEF are not responsive to conservative medical treatment and the clinical outcome is dismal (5). Consensus documents have recently been released advocating for institutions with multidisciplinary care teams to improve the outcomes of patients with severe respiratory disease. Thus, a multidisciplinary approach is required to increase the efforts to preserve the life of IPA patients with severe complications.
The diagnosis of IPA in our patient was confirmed by chest CT scan, serum GM test and sputum direct smear at the first week in ICU stay. Although timely antifungal therapy was given, prolonged neutropenia, caused by aplastic anemia may be a predisposing factor for the development of invasive aspergillosis. The aspergillus penetrated the mucosa leading to obstruction of the right main bronchus and subsequently to the TEF. The fistula was noticed during high-flow nasal cannula oxygen therapy with the symptom of abdomen distension and confirmed by bronchoscopy.
Early detection by routine bronchoscopy is crucial in the diagnosis and treatment of IPA, because some patients with IPA may have normal chest X-rays as a result of inadequate inflammatory response. Tracheobronchial disease is the earliest and most common manifestation of IPA, usually diagnosed when ulcerations or pseudomembranes are noted on surveillance bronchoscopy, or on diagnostic bronchoscopy for symptoms such as fever, cough and wheezing (6). TEF is identified by an abnormal connection (fistula) between the esophagus and trachea. With the development of endoscopic techniques, the treatment of TEF has made marked progress (7). Bronchoscopic intervention provides a good choice to palliate symptoms and reconstruct the airway and esophagus instead of surgical intervention especially in the patients with critical status. Airway stenting via endoscopic techniques is by far the optimal clinical option (8,9). The patients we reported were the one with aplastic anemia who had a very low level of platelet (2×109/L) which might easily cause bleeding by surgery. More important, stomal leak occurs frequently in patients with infectious diseases, malnutrition or immunosuppression. So airway stent is the best choice to this patient which is effective to seal the fistula and prevent the leakage of liquid or gas.
In aggregate, we reported the first case of TEF induced by IPA which was cured by multidisciplinary care. Antifungal and bronchoscopic intervention was provided during ICU stay. Early detection and treatment of the disease did improve the outcomes. This experience is worth learning.
Acknowledgements
We appreciate Zhongheng Zhang from Department of Critical Care Medicine in Jinhua Municipal Central Hospital for revising the language of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008;46:327-60. [Crossref] [PubMed]
- Hürtgen M, Herber SC. Treatment of malignant tracheoesophageal fistula. Thorac Surg Clin 2014;24:117-27. [Crossref] [PubMed]
- Kapelushnik J, Springer C, Naparstek E, et al. Tracheoesophageal fistula induced by aspergillus infection following bone marrow transplantation. Pediatr Pulmonol 1994;17:202-4. [Crossref] [PubMed]
- Cadena J, Thompson GR 3rd, Patterson TF. Invasive Aspergillosis: Current Strategies for Diagnosis and Management. Infect Dis Clin North Am 2016;30:125-42. [Crossref] [PubMed]
- Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax 2015;70:270-7. [Crossref] [PubMed]
- Wu N, Huang Y, Li Q, et al. Isolated invasive Aspergillus tracheobronchitis: a clinical study of 19 cases. Clin Microbiol Infect 2010;16:689-95. [Crossref] [PubMed]
- Ke M, Wu X, Zeng J. The treatment strategy for tracheoesophageal fistula. J Thorac Dis 2015;7:S389-97. [PubMed]
- Klotz LV, Gesierich W, Schott-Hildebrand S, et al. Endobronchial closure of bronchopleural fistula using Amplatzer device. J Thorac Dis 2015;7:1478-82. [PubMed]
- Ho AM, Dion JM, Wong JC. Airway and Ventilatory Management Options in Congenital Tracheoesophageal Fistula Repair. J Cardiothorac Vasc Anesth 2016;30:515-20. [Crossref] [PubMed]