Use of extracorporeal membranous oxygenator in transcatheter aortic valve replacement
Introduction
Aortic stenosis (AS) is the most common form of valvular heart disease that predominantly affects elderly patients. Its prevalence is estimated at 4.6% in patients greater than 75 years of age (1). Patients with severe AS who develop symptoms have a very poor prognosis with significant decrease in survival and a 50% mortality within 2 years without treatment (2). The operative risk is elevated during conventional aortic valve replacement with a mortality ranging up to 30% in patients with advanced heart failure (3-6). After the introduction of transcatheter aortic valve replacement (TAVR) by Cribier et al. (7) in 2002, the superiority of TAVR compared with medical therapy for patients considered too high risk for surgery has been established. TAVR has become more attractive as the appropriate alternative for elderly patients with very high surgical risk (4,5,8-11). Considering the prognosis of patients who are not candidates for TAVR nor conventional aortic valve replacement have mortality rate at 6 months is 31.8% and at 2 years 53.4% (6,12), Leon et al. and Reiss et al. (13,14) concluded in the PARTNER trial that “TAVR should be the new standard of care for patients with AS who are not suitable candidates for surgery”. Nowadays, TAVR continues to grow beyond those populations originally studied, to include those with severe left ventricular dysfunction and those with failing surgical homografts (valve-in-valve TAVR) (15,16). Although, TAVR expands the options for patients with severe AS and is less-invasive alternative in high-risk frail and decompensated patients, it remains a complex procedure that may result in serious complications (e.g., severe aortic regurgitation, major bleeding, device embolization, coronary occlusion, and aortic dissection). While these complications are uncommon, they may precipitate sudden hemodynamic collapse necessitating cardiopulmonary bypass (CPB) or other mechanical support (17,18).
Use of CPB in TAVR procedures
Mechanical circulatory support (MCS) devices were used in nearly 10.6% of the patients who underwent TAVR procedures, among these CPB was used between 1.2% to 6.6% of TAVR cases (8,18,19). Drews et al. (20), however, reported increase of the CPB use to 13% of these very high-risk patients during valve implantation. It also has been shown that using MCS in TAVR procedures was associated with significantly high rates of mortality, complications, and increased length and cost of hospitalization (8). The use of MCS was also identified as an independent predictor of increased early and late mortality (21). Furthermore, it was also reported that CPB recipients had the highest 1-year mortality rate compared to patients who required intra-aortic balloon pump (IABP) and no support at all: 1-year mortality rate was 52.8% in those who received MCS emergently versus planned MCS of 40.3% versus no MCS of 21.6% (19).
Not all patients electively put on CPB during the procedure need the support. However, elective CPB should be considered in patients with severe cardiogenic shock, poor left ventricular function, or enlarged right ventricle with severe pulmonary hypertension (20). Its elective use will increase the safety in critically ill patients in order to maintain hemodynamic stability during the phases of rapid pacing and to eliminate manual cardiopulmonary resuscitation as the postoperative course of these patients is unfavorable (20).
CPB is also used as an intraoperative emergent method to rescue patients from myocardial collapse as a consequence of the most severe TAVR complications and allows time to perform a thorough diagnostic evaluation and facilitate a safe definitive treatment of the complication (18). Intraoperative emergent complications needing CPB might include severe paravalvular leak in patients with depressed left ventricular function, severe diastolic dysfunction, or significant mitral regurgitation. The ability of these patients to compensate for acute severe aortic insufficiency may be compromised (22-25). The use of CPB allows time for a full assessment of the leak and either re-ballooning of the prosthesis or preparation of a second device for valve-in-valve treatment. CPB also can be used in cases of coronary malperfusion, or severe bleeding at the apex of the left ventricle which allows decompression of the ventricle to facilitate a safe primary repair. The hemodynamic support provide by CPB is not without potential harm as it is well documented in the literature that CPB is associated with undesirable side effects, e.g., activation of inflammatory mediators, increased pulmonary vascular resistance, platelet activation, coagulopathy and impaired renal function (26-29).
Use of extracorporeal membranous oxygenator (ECMO) as an alternative to CPB in TAVR procedures
The advances in ECMO technology and the improvement of commercially available percutaneous cannulas of different sizes and lengths in the complete implantation sets make ECMO at present day a more powerful resuscitation tool (30,31). It is also relatively less expensive than some forms of MCS (32). Additionally, it is easier to transport the patient with ECMO support or to use it bedside if necessary. Miniaturized ECMO systems can be highly effective and safe for the initiation of emergency ECMO while performing cardiopulmonary resuscitation (e-CPR) in the cardiac catheterization laboratories. Especially for patients in need of cardiac surgery, transfer to extracorporeal assistance can be more easily processed by using miniaturized ECMO systems (30). Furthermore, ECMO provides both cardiac and pulmonary support for patients for some duration until they recover from the complications or a decision is made for definite operative plans. The unit can also provide mild hypothermia for cerebral protection in the event of complete prolonged hemodynamic collapse (33).
Use of ECMO as emergency rescue
Along with some case reports regarding using ECMO as emergency rescue (34-36), there are four papers (22-24,30) reporting series of multiple patients these are listed in Table 1 (1), in these series the using of ECMO permitted to procedure completion in 44–66%, support to surgery in 33–56% and survival to discharge in 44–75% of cases. Over all in the cohort study published by Husser et al. (22): the veno-arterial extracorporeal membrane oxygenation (VA-ECMO) cohort was significantly higher risk (median logistic EuroSCORE 26% vs. 15%). They concluded that emergent implantation of VA-ECMO for circulatory support appears to be safe and feasible to stabilize the patient for further treatment (22). Both Seco et al. and Husser et al. concluded that VA-ECMO may potentially minimize the effect of TAVR complications (22,23). Most common reasons for emergent peri-procedural initiation of ECMO were: (I) ventricular perforation; (II) hemodynamic instability; (III) refractory cardiogenic shock; and (IV) hemodynamic deterioration due to ventricular arrhythmia.
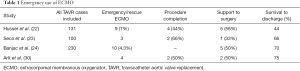
Full table
These outcomes are comparable to the literature about using CPB for emergency support of intraoperative TAVR complication. Eggebrecht et al. (37) reported 12 patients (4%) of their series required emergent CPB; all 4 needed surgical conversion with a resulting 30-day survival of 52%. Roselli et al. (18) also reported a single-centre series, 12 (4%) of 303 patients undergoing TAVR required emergency CPB following complications resulting in hemodynamic collapse. In three patients a period of resuscitation with CPB was sufficient for recovery, while nine required complication-specific procedures (e.g., valve-in-valve TAVR, conversion to open procedure and SAVR). Seven patients required additional circulatory support, five via IABP, and two via VA-ECMO. Thirty-day mortality was 16% with only 45% survival at 12 months. This is far lower survival than in TAVR patients not requiring emergency CPB (19).
Use of ECMO as prophylactic measure
There is little in the literature reported about using prophylactic support with VA-ECMO with only two papers (22,23) reporting series of multiple patients. These are listed in Table 2. In a cohort study about ECMO use in nine TAVR patients, the authors noted that preemptive use of ECMO in selected high risk patients was associated with improved procedural success (100%) and 30-day survival to discharge of 100%. They concluded that prophylactic strategy may be suitable in the following scenario: severely impaired left ventricular function, slow recovery from rapid left ventricular pacing during testing of a pacemaker, high vasopressor requirements during general anesthesia or concomitant high risk PCI (22). Seco et al. (23) reported the same results in their series of eight patients. These outcomes are also comparable to the published data about using CPB in patients of very high risk (logistic EuroSCORE 59%, STS 35%). Overall technical success (94%) and peri-procedural complications were comparable to the standard TAVR cohort, suggesting planned ECMO may be a feasible adjunct in high-risk cases that may otherwise have been declined TAVR (20).

Full table
Given that TAVR patients are often frail and decompensated, early signs of hemodynamic instability during anesthetic induction may be predictive of subsequent problems and dictate additional measures to be initiated for stabilization (38).
Conclusions
The outcomes of using ECMO as prophylactic are comparable with conventional TAVR patients, whereas requirement for emergency VA-ECMO was associated with significantly lower procedural success and survival. Using of ECMO could replace CBP used as prophylaxis in high risk patients undergoing TAVR insertion and would be used to stabilize patients in cases of hemodynamic instability with or without ischemic changes, with comparable results to CPB use.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Ross J Jr, Braunwald E. Aortic stenosis. Circulation 1968;38:61-7. [Crossref] [PubMed]
- Brown JM, O'Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [Crossref] [PubMed]
- Osten MD, Feindel C, Greutmann M, et al. Transcatheter aortic valve implantation for high risk patients with severe aortic stenosis using the Edwards Sapien balloon-expandable bioprosthesis: a single centre study with immediate and medium-term outcomes. Catheter Cardiovasc Interv 2010;75:475-85. [PubMed]
- Thielmann M, Wendt D, Eggebrecht H, et al. Transcatheter aortic valve implantation in patients with very high risk for conventional aortic valve replacement. Ann Thorac Surg 2009;88:1468-74. [Crossref] [PubMed]
- Ben-Dor I, Pichard AD, Gonzalez MA, et al. Correlates and causes of death in patients with severe symptomatic aortic stenosis who are not eligible to participate in a clinical trial of transcatheter aortic valve implantation. Circulation 2010;122:S37-42. [Crossref] [PubMed]
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Singh V, Patel SV, Savani C, et al. Mechanical circulatory support devices and transcatheter aortic valve implantation (from the National Inpatient Sample). Am J Cardiol 2015;116:1574-80. [Crossref] [PubMed]
- Walther T, Simon P, Dewey T, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 2007;116:I240-5. [Crossref] [PubMed]
- Webb JG, Altwegg L, Boone RH, et al. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation 2009;119:3009-16. [Crossref] [PubMed]
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007;50:69-76. [Crossref] [PubMed]
- Unbehaun A, Pasic M, Buz S, et al. Transapical aortic valve implantation in patients with severely depressed left ventricular function. J Thorac Cardiovasc Surg 2012;143:1356-63. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Reiss GR, Smith CR. PARTNER B: where will it take us? Semin Thorac Cardiovasc Surg 2011;23:85-6. [Crossref] [PubMed]
- Elhmidi Y, Bleiziffer S, Deutsch MA, et al. Transcatheter aortic valve implantation in patients with LV dysfunction: impact on mortality and predictors of LV function recovery. J Invasive Cardiol 2014;26:132-8. [PubMed]
- Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 2012;126:2335-44. [Crossref] [PubMed]
- Allen CJ, Duncan AM, Moat NE, et al. TAVI – assessing the need for circulatory support. Br J Cardiol 2014;21:96-7.
- Roselli EE, Idrees J, Mick S, et al. Emergency use of cardiopulmonary bypass in complicated transcatheter aortic valve replacement: importance of a heart team approach. J Thorac Cardiovasc Surg 2014;148:1413-6. [Crossref] [PubMed]
- Shreenivas SS, Lilly SM, Szeto WY, et al. Cardiopulmonary bypass and intra-aortic balloon pump use is associated with higher short and long term mortality after transcatheter aortic valve replacement: a PARTNER trial substudy. Catheter Cardiovasc Interv 2015;86:316-22. [Crossref] [PubMed]
- Drews T, Pasic M, Buz S, et al. Transcatheter aortic valve implantation in very high-risk patients with EuroSCORE of more than 40%. Ann Thorac Surg 2013;95:85-93. [Crossref] [PubMed]
- Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080-90. [Crossref] [PubMed]
- Husser O, Holzamer A, Philipp A, et al. Emergency and prophylactic use of miniaturized veno-arterial extracorporeal membrane oxygenation in transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2013;82:E542-51. [PubMed]
- Seco M, Forrest P, Jackson SA, et al. Extracorporeal membrane oxygenation for very high-risk transcatheter aortic valve implantation. Heart Lung Circ 2014;23:957-62. [Crossref] [PubMed]
- Banjac I, Petrovic M, Akay MH, et al. Extracorporeal Membrane Oxygenation as a Procedural Rescue Strategy for Transcatheter Aortic Valve Replacement Cardiac Complications. ASAIO J 2016;62:e1-4. [Crossref] [PubMed]
- Makdisi G, Makdisi PB, Wang IW. New horizons of non-emergent use of extracorporeal membranous oxygenator support. Ann Transl Med 2016;4:76. [PubMed]
- Pintar T, Collard CD. The systemic inflammatory response to cardiopulmonary bypass. Anesthesiol Clin North America 2003;21:453-64. [Crossref] [PubMed]
- Clive Landis R, Murkin JM, Stump DA, et al. Consensus statement: minimal criteria for reporting the systemic inflammatory response to cardiopulmonary bypass. Heart Surg Forum 2010;13:E116-23. [PubMed]
- Momeni M, Carlier C, Baele P, et al. Fibrinogen concentration significantly decreases after on-pump versus off-pump coronary artery bypass surgery: a systematic point-of-care ROTEM analysis. J Cardiothorac Vasc Anesth 2013;27:5-11. [Crossref] [PubMed]
- Belhaj A. Actual knowledge of systemic inflammation reaction during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov 2012;7:165-9. [Crossref] [PubMed]
- Arlt M, Philipp A, Voelkel S, et al. Early experiences with miniaturized extracorporeal life-support in the catheterization laboratory. Eur J Cardiothorac Surg 2012;42:858-63. [Crossref] [PubMed]
- Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015;7:E166-76. [PubMed]
- Pagani FD, Lynch W, Swaniker F, et al. Extracorporeal life support to left ventricular assist device bridge to heart transplant: A strategy to optimize survival and resource utilization. Circulation 1999;100:II206-10. [Crossref] [PubMed]
- Stub D, Bernard S, Duffy SJ, et al. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation 2011;123:1428-35. [Crossref] [PubMed]
- Kuhn EW, Madershahian N, Rudolph TK, et al. Catheter Insertion via Extracorporeal Membrane Oxygenation Cannula during Transcatheter Aortic Valve Implantation. Thorac Cardiov Surg reports. Available online: https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-0036-1572512.pdf
- Fabris E, Perkan A, Rauber E, et al. Bilateral coronary obstruction in high-risk transcatheter aortic valve-in-valve implantation: When procedural strategy counts. Int J Cardiol 2016;203:672-4. [Crossref] [PubMed]
- Eric van belle. TAVR: A case of cardiac arrest at start of general anesthesia. Available online: http://caci.org.ar/docs/van-belle-eric-20140520-1520-room-242ab.pdf
- Eggebrecht H, Mehta RH, Kahlert P, et al. Emergent cardiac surgery during transcatheter aortic valve implantation (TAVI): insights from the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry. EuroIntervention 2014;10:975-81. [Crossref] [PubMed]
- Tam DY, Jones PM, Kiaii B, et al. Salvaging catastrophe in transcatheter aortic valve implantation: rehearsal, preassigned roles, and emergency preparedness. Can J Anaesth 2015;62:918-26. [Crossref] [PubMed]


