Future perspectives in cancer immunotherapy
Advances in cancer immunotherapy in recent years, with the success of checkpoint inhibition in particular, have caused a paradigm shift in cancer management. After many years of extensive research, the basic mechanisms behind immune surveillance and tumor evasion have been elucidated and that lead to the development of effective immunotherapeutic strategies. In early clinical trials, remarkable responses have been reported but these involved a minority of patients. On the other hand, with the use of immunotherapeutic agents we witnessed an uncommon toxicity profile and we were faced with different kinetics in clinical responses, as well. In order to address these issues, we need to select the patients that will benefit the most from specific immunotherapeutic modalities, with the use of appropriate biomarkers.
Although today’s enthusiasm regarding cancer immunotherapy was generated from the results of CTLA-4 and PD-1/PD-L1 inhibition in melanoma patients, several other checkpoint molecules are under investigation, such as TIM-3 and LAG-3. TIM-3, as a checkpoint inhibitor, suppresses effector T cell activation, whereas LAG-3 acts by binding to MHC molecules and also inhibits T cell activation and proliferation (1,2). LAG-3 is co-expressed with PD-1 on T cells, making it a suitable candidate for a combinatorial approach with anti-PD-1 agents. Antibodies against TIM-3 and LAG-3 are under clinical investigation showing encouraging efficacy. Several other targets of host immunity are currently being evaluated in the pre-clinical and clinical settings, including inhibitory (IDO1, B7-H3, B7-H4, VISTA, ICOS, KIR and TIGIT) and stimulatory (OX40, 4-1BB and GITR) molecules (3).
Vaccines, either preventive or therapeutic, against tumor specific or associated antigens are a promising immunotherapeutic strategy, as well. Sipuleucel-T was the first and only approved vaccine for metastatic castration-resistant prostate cancer, which confers a modest survival benefit to patients (4). GVAX, a whole-cell vaccine, showed encouraging results only in combination with CRS-207, a Listeria monocytogenes-expressing mesothelin vaccine, in advanced pancreatic cancer patients (5). Nevertheless, the majority of vaccines used as monotherapy to treat cancer had failed and this depicts the need to develop novel vaccination strategies, using them in combination with adjuvant or other immunotherapeutic agents, such as checkpoint inhibitors, in order to address tumor-induced immunosuppression (6).
Adoptive cell transfer of tumor-infiltrating lymphocytes (TILs) was traditionally an attractive immunotherapeutic modality. Such TILs were isolated from the patient’s tumor, expanded ex vivo and re-infused in combination with IL-2 (7). On the other hand, genetic modification and characterization of T cells with chimeric antigen receptors (CARs) allow the recognition of specific tumor antigens by T cells and the elimination of tumor cells. CARs are generated by fusing the antigen-binding region of a monoclonal antibody (mAb) to the membrane-spanning and intracellular-signaling domains, including CD28, 4-1BB and CD137 co-stimulatory molecules. They have recently shown impressive results in patients with acute and chronic lymphoblastic leukemia treated with adoptive transfer of CD19-directed autologous T cells. Recent successes suggest that the modification of T cells with CARs could be a powerful tool for developing safe and effective cancer immunotherapeutic approaches. This strategy can now be further improved and applied on patients with solid malignancies by sequencing the tumor’s whole exome and identifying specific and unique neoantigens. Following that, autologous T cells can be engineered to express a CAR against the identified neoantigen. These cells, after expansion and re-infusion, may produce significant clinical responses (8).
Since tumor-host interactions are complex, the strategy to target only one of them may be inadequate. Therefore, immunotherapy in combination with other therapeutic strategies or the administration of different immunotherapeutic modalities together may act synergistically and might restore immune responses more efficiently. The combination of ipilimumab with nivolumab in melanoma showed impressive results leading to accelerated approval from FDA in September of 2015 (9). To date, this is the first and only approved immunotherapeutic combination and is currently being tested in other tumors (10). Several other combinations with checkpoint inhibitors, such as TIM-3, LAG-3 and OX-40, exhibited impressive results in pre-clinical models and are being tested in early-phase trials. Growing knowledge regarding the effects of chemotherapy, radiotherapy and targeted therapy on immunomodulation allowed the design of other immunotherapy combinatorial approaches showing very encouraging results in phase I trials (11).
The aforementioned immunotherapeutic strategies are the result of the application of the accumulated knowledge regarding the tumor-immune system interactions. These approaches are totally different compared to conventional therapies, in terms of efficacy or toxicity, there is therefore a need for the development of new tools for the conduct of such research. Durable responses and the novel kinetics of response observed with immunotherapeutic agents require the definition of new efficacy endpoints in clinical trials. The introduction of immune-related response criteria (irRC) and exploratory endpoints, such as landmark survival, and their adoption from regulatory authorities will be essential for better and faster reporting of efficacy in future clinical trials (12-14).
In order to increase the percentage of patients responding to immunotherapeutic agents, reliable biomarkers are needed. The first immune biomarker used in immune-oncology clinical trials is tumor PD-L1 protein expression that helps identify patients, which are more likely to benefit from anti-PD-1/PD-L1 agents. However, due to the fact that PD-L1 protein expression is inducible and the existence of several assays with different cut-off points for positivity, their utility is controversial and still under discussion (15). In addition to PD-L1, other potential biomarkers that can identify responders include the tumor immune infiltrate score (immunoscore), consisting of the frequency of CD3+ and CD8+ cells (16) or the tumor mutational load that may indicate higher rates of host immune responses to several neoantigens, thus leading to increased responses to checkpoint inhibition (17,18).
Most of the clinical success in the immunotherapy landscape is still based on the universal checkpoint modulatory antibodies, but this success is anticipated to expand to other modalities, as well. It is important to remember that immune-oncology agents across different modalities can show distinct clinical efficacy and safety profiles, but also, potential synergistic effects. For combination therapies, investigations may be designed with an understanding of the underlying mechanisms of action, the expected clinical profiles and the potential synergistic activity of the agents involved (19). The resulting effect of these combinations may well exceed expectations for individual agents in terms of pharmacokinetics and pharmacodynamics, which renders combinations very appealing for the clinical trial arena. Ultimately, such combinations may hopefully revive the potential for cure, either at a functional level by turning cancer into a controllable chronic disease (similar to the way retroviral drug combinations work) or by the true eradication of the disease, which may indeed now be a realistic goal for a clinically significant number of advanced cancer patients (19).
In conclusion, novel immunotherapeutic agents, used alone or in combination with conventional therapies and demonstrating unique survival benefits, have the potential to transform cancer to a chronic disease and this is now the reality for many cancer patients treated with such agents. However, we need to further improve our immunotherapeutic modalities in order to maximize efficacy and minimize toxicity and this could only be achieved through well-designed clinical trials. Indeed, there are countless studies in several tumors and in different settings that are in progress today and Table 1 shows that by citing a list of ongoing trials only in the field of breast cancer. The immune-oncology field is rapidly evolving and current excitement is not expected to dissipate but rather continue to increase in the following years, as new effective strategies keep constantly emerging.
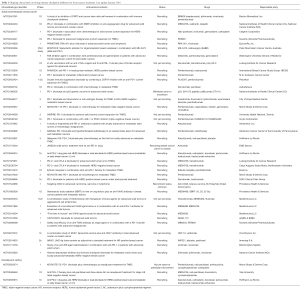
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012;72:917-27. [Crossref] [PubMed]
- Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010;207:2187-94. [Crossref] [PubMed]
- Mandal R, Chan TA. Personalized Oncology Meets Immunology: The Path toward Precision Immunotherapy. Cancer Discov 2016;6:703-13. [Crossref] [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [Crossref] [PubMed]
- Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015;33:1325-33. [Crossref] [PubMed]
- van der Burg SH, Arens R, Ossendorp F, et al. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 2016;16:219-33. [Crossref] [PubMed]
- Yang JC, Rosenberg SA. Adoptive T-Cell Therapy for Cancer. Adv Immunol 2016;130:279-94. [Crossref] [PubMed]
- Tran E, Ahmadzadeh M, Lu YC, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 2015;350:1387-90. [Crossref] [PubMed]
- Spain L, Larkin J. Combination immune checkpoint blockade with ipilimumab and nivolumab in the management of advanced melanoma. Expert Opin Biol Ther 2016;16:389-96. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 2010;102:1388-97. [Crossref] [PubMed]
- Hoos A, Wolchok JD, Humphrey RW, et al. CCR 20th Anniversary Commentary: Immune-Related Response Criteria--Capturing Clinical Activity in Immuno-Oncology. Clin Cancer Res 2015;21:4989-91. [Crossref] [PubMed]
- Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol 2016;34:1510-7. [Crossref] [PubMed]
- Budczies J, Bockmayr M, Denkert C, et al. Pan-cancer analysis of copy number changes in programmed death-ligand 1 (PD-L1, CD274) - associations with gene expression, mutational load, and survival. Genes Chromosomes Cancer 2016;55:626-39. [Crossref] [PubMed]
- Kirilovsky A, Marliot F, El Sissy C, et al. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med 2016;14:73. [Crossref] [PubMed]
- Dudley JC, Lin MT, Le DT, et al. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res 2016;22:813-20. [Crossref] [PubMed]
- Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 2016;15:235-47. [Crossref] [PubMed]


