Gene expression profile of THP-1 cells treated with heat-killed Candida albicans
Introduction
In healthy individuals, Candida albicans (C. albicans) is harmless and acts as a part of the normal flora in alimentary tract and mucocutaneous membranes. However, in immune comprised individuals, C. albicans can cause opportunistic infections named candidiasis, with symptoms ranging from superficial lesions to fatal systemic disease (1).
Innate immunity is the first line of immune system to defense against C. albicans. After sensing the pathogen-associated molecular patterns (PAMPs) on surface of C. albicans, the innate immune cells (e.g., monocyte, macrophage, and dendritic cell) are immediately activated and the inflammatory response is initiated. In the infection site, activated innate immune cells release inflammatory factors, such interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α), which can promote the clearance of C. albicans in early phase (2,3). In addition, the antigens of C. albicans are processed and presented to T cells by antigen presenting cells (APCs), and then the adapt immune response against C. albicans is initiated. The type and strength of adapt immune response are shaped and programmed by APCs released cytokines, such as IL-12, IL-23, transforming growth factor (TGF)-β (4). Actually, some of the innate immune cells (e.g., monocytes, macrophages, and dendritic cells) are also acted as APCs in the immune response. Taken together, the activation of innate immune cells is a crucial step in anti-C. albicans innate and adapt immune responses.
Currently, the molecular mechanisms under the production of inflammatory factors and cytokines are not fully understood. Two studies have investigated the gene expression profile of monocyte treated with C. albicans¸ but biological information analysis was not performed. To better understand the mechanisms of innate immune cell activation, we analyzed the gene expression profile of heat-killed C. albicans stimulated THP-1 cell, a widely used monocytic human cell line.
Methods
Cell culture and stimulation
THP-1 cells were obtained from the American Type Culture Collection (Manassas, VA). Log-phase cells were cultured in RPMI 1640 medium (HyClone, Logan, UT) containing 10% FBS (v/v) (Gibicol, Carlsbad, CA, USA), 100 U/mL penicillin-streptomycin (Mediatech). The cells were cultured at the concentration of 106 in a 6-well plate.
C. albicans was suspended in a PBS solution, washed three times and heat killed at 100 degrees for 30 minutes. Heat-killed C. albicans was added to THP-1 cells at a ratio of 1:1. PBS solution was used as a control. Nine hours later, cells were harvest and RNA was extracted.
Microarray analysis
The RNA of heat-killed C. albicans stimulated THP-1 cells was extracted by Trizol (Invitrogen, Carlsbad, CA) following manufacturer’s protocols. RNA quantity and quality were measured using NanoDrop ND-1000. RNA integrity was assessed by a standard denaturing agarose gel electrophoresis.
The Whole Human Genome Oligo Microarray was a broad view that represents all known genes and transcripts in the human genome. Sequences were compiled from a broad source survey, and then verified and optimized by alignment to the assembled human genome. The microarray analysis was performed and analyzed as previously described (5). Briefly, RNA from each sample was linearly amplified and labeled with Cy3-UTP. An RNeasy Mini Kit (Qiagen) was used to purify the labeled cRNAs. The specific activity and concentration of the labeled cRNAs (pmol Cy3/μg cRNA) were measured by NanoDrop ND-1000. A total of 1 µg of each labeled cRNA was fragmented by adding 11 µL 10× Blocking Agent and 2.2 µL of 25× Fragmentation Buffer, then the mixture was heated at 60 °C for 30 min, and 55 μL 2× GE Hybridization buffer was then added to dilute the labeled cRNA. A total of 100 μL of hybridization solution was dispensed into the gasket slide and assembled to the gene expression microarray slide. The slides were put in an Agilent Hybridization Oven incubated for 17 hours at 65 °C. The hybridized arrays were washed, fixed and scanned with using the Agilent DNA Microarray Scanner (part number G2505C).
Data analysis
We analyzed the acquired array images using Agilent Feature Extraction software (version 11.0.1.1). Quantile normalization and subsequent data analysis were performed using the GeneSpring GX v12.1 software package (Agilent Technologies). Genes that at least 3 out of 6 samples have flags in detected were used for further analysis. Differentially expressed genes were defined as change folds more than 2 and with statistical significance (P<0.05). Scatter plot and heatmap were used to depict the differentially expressed genes. GO analysis and Pathway analysis were performed in the standard enrichment computation method.
Results
RNA quantity and quality
As shown in Figure 1, RNA extracted from THP-1 cells was intact. The concentration of RNA ranged from 617 to 1,177 ng/μL.
Gene expression microarrays
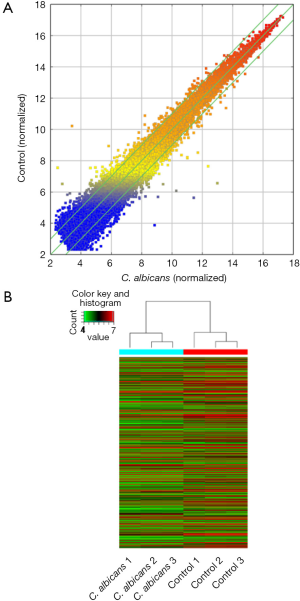
We found 1,070 differentially expressed genes, of which 355 were up-regulated and 715 were down-regulated [for more details, please contact the corresponding authors or Dr. Zhi-De Hu (hzdlj81@163.com)]. Scatter plot of detected genes is showed in Figure 2A, and a heatmap of differentially expressed genes is showed in Figure 2B. Table 1 lists top ten differentially expressed genes.
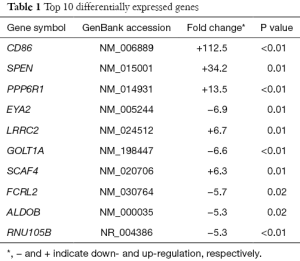
Full table
Gene ontology (GO) and pathway analysis
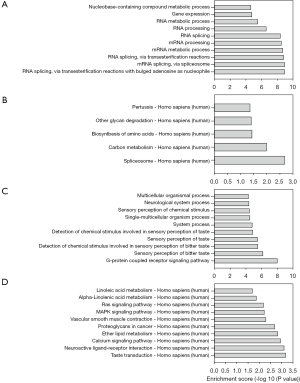
GO analysis showed that up-regulated genes were particularly involved in biological process of RNA processing (Figure 3A). Consistent with the GO results, pathway analysis also indicated that the up-regulated genes were critically involved in pathway of spliceosome and carbon metabolism (Figure 3B). In the case of down-regulated genes, the most significant involved immune-related biological process was G-protein coupled receptor signaling pathway (Figure 3C), followed by some terms of sensory perception of taste. Among the top ten down-regulated gene involved pathways, three were closely related to immune response (calcium signaling pathway, MAPK signaling pathway and Ras pathway, Figure 3D).
Discussion
Response against C. albicans is initiated by innate immune cells. The strength, duration, and type of the immune response against C. albicans are largely affected by these innate immune cells. Therefore, it is interesting and valuable to investigate the mechanisms under the activation of innate immune cells. In present study, we depict the gene expression profile of heat-killed C. albicans stimulated THP-1 cell, a widely used monocytic human cell line. A total of 355 genes were up-regulated and 715 genes were down-regulated.
Compared with previous study that investigated the gene expression profile of THP-1 cells stimulated with C. albicans (6), one strength of present study is that we performed GO and pathway analysis to systematically identify biological connections of differentially expressed genes, as well as the pathways associated with the immune response against C. albicans. We found that the differentially expressed genes were involved in many pathways related to immune response. For example, we found that the down-regulated genes were involved in mitogen-activated protein kinases (MAPKs) signaling pathway. MAPK is a family of serine/threonine kinases that participates in pathogen-derived signal transduction events. Three members of this family have been identified: p38 MAPK, extracellular signal-regulated kinases (ERK, also termed classic MAPK pathway), and c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK). Previous studies have shown that C. albicans could activate classic MAPK signaling pathway in J744 cell line (7) and human monocytes (8), which could promote the phagocytosis of C. albicans and inhibit IL-12 production. In addition, Dectin-1 has been reported to be one of the pattern recognition receptor in innate immune sensing of C. albicans (9). Jia et al. (10) found that C. albicans could activate ERK through Dectn-1 and H-Ras, which is involved in the releasing of inflammatory factors. We found that the genes downregulated in MAPK pathway contain MAP kinase-specific phosphatase (MKP), protein tyrosine phosphatases (PTPs) and protein kinase C (PKC) et al. It has been reported that MKP and PTP were critically involved in the regulation of MAPK pathway (11). Therefore, we speculate that the down-regulation of MKP and PTP may be attributed to the activation of MAPK pathway, which may promote the production of inflammatory factors.
One weakness of the present study is that we only depict the gene expression profile of heat killed C. albicans treated THP-1 cells using microarray. Reverse transcription PCR (RT-PCR) and western blot were not used to validate the microarray results. Because false positive or negative results are not avoidable in microarray analysis, further studies are needed to validate the results of the microarray.
In summary, present study depicted the gene expression profile of heat killed C. albicans treated THP-1 cells and identified major pathways involved in the immune response against C. albicans. These pathways are potential targets for developing anti-C. albicans agent.
Acknowledgements
The authors would like to thank Kangchen Bio-tech Inc. (Shanghai, China) for technical assistance.
Funding: This work was supported by a grant from the National Natural Science Foundation of China (Grant Number 81302541; 81471608).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional ethic review board and informed consent was obtained from all patients.
References
- Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence 2013;4:119-28. [Crossref] [PubMed]
- Gil ML, Gozalbo D. Role of Toll-like receptors in systemic Candida albicans infections. Front Biosci (Landmark Ed) 2009;14:570-82. [Crossref] [PubMed]
- Gozalbo D, Gil ML. IFN-gamma in Candida albicans infections. Front Biosci (Landmark Ed) 2009;14:1970-8. [Crossref] [PubMed]
- Romani L. Immunity to fungal infections. Nat Rev Immunol 2011;11:275-88. [Crossref] [PubMed]
- Zhou J, Gao J, Liu Y, et al. Human atrium transcript analysis of permanent atrial fibrillation. Int Heart J 2014;55:71-7. [Crossref] [PubMed]
- Barker KS, Liu T, Rogers PD. Coculture of THP-1 human mononuclear cells with Candida albicans results in pronounced changes in host gene expression. J Infect Dis 2005;192:901-12. [Crossref] [PubMed]
- Ibata-Ombetta S, Jouault T, Trinel PA, et al. Role of extracellular signal-regulated protein kinase cascade in macrophage killing of Candida albicans. J Leukoc Biol 2001;70:149-54. [PubMed]
- Tang N, Liu L, Kang K, et al. Inhibition of monocytic interleukin-12 production by Candida albicans via selective activation of ERK mitogen-activated protein kinase. Infect Immun 2004;72:2513-20. [Crossref] [PubMed]
- Netea MG, Brown GD, Kullberg BJ, et al. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 2008;6:67-78. [Crossref] [PubMed]
- Jia XM, Tang B, Zhu LL, et al. CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J Exp Med 2014;211:2307-21. [Crossref] [PubMed]
- Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 2000;12:186-92. [Crossref] [PubMed]