Design of nutrition trials in critically ill patients: food for thought
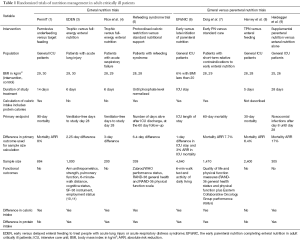
We would like to thank Drs. Casaer and Van den Berghe for their thoughtful editorial on our article “Permissive underfeeding or standard enteral feeding in critically ill adults” published in the New England Journal of Medicine on June 18, 2015 (1,2). Over the last few years, several large clinical trials have added immensely to our knowledge regarding nutritional support of critically ill patients. Table 1 summarizes and contrasts eight recent multicenter trials which compared different doses of enteral nutrition (1,3-5), or enteral versus parenteral nutrition (6-9).
Full table
PermiT and other trials showed no difference in outcomes in patients receiving restricted versus full caloric intake. Drs. Casaer and Van den Berghe raise many important questions regarding these trials: Is mortality an appropriate primary endpoint for nutrition trials? Do we need larger trials to detect smaller treatment effect? Should we use different endpoints than mortality? How about biomarkers? How generalizable are the results of PermiT to normal weight or underweight patient populations? Are specific patient groups more likely to be nutrition-responsive?
Is mortality an appropriate primary endpoint for nutrition trials?
While early enteral feeding in critically ill patients has been shown in systematic reviews to reduce mortality (12), the association between caloric intake and mortality is less clear, with several observational studies reporting conflicting results (13-16). In a previous 2×2 factorial design trial of hypocaloric feeding and intensive insulin therapy, we demonstrated lower hospital mortality with hypocaloric intake, although this was a secondary endpoint (17). There is sufficient pathophysiological evidence to suggest that caloric intake may alter many important biological processes that may affect mortality. Caloric restriction has been shown to prolong life span in several species (18,19), promote mammalian cell survival (20) and improve biomarkers of longevity in humans (21). These effects may be mediated through the effect of caloric restriction on reducing metabolic rate, oxidative stress (22), and mitochondrial free radical generation (23) improving insulin sensitivity and myocardial ischemia tolerance (24) and modifying neuroendocrine and sympathetic nervous system function (19). These findings buttress the equipoise regarding the effect of caloric intake on mortality, which was the basis for PermiT and other trials. As reflected in Table 1, several trials used mortality (at 28- to 90-day) as the primary endpoint; others used intensive care unit (ICU) length of stay, ventilator free-days or nosocomial infections. Mortality is an objective outcome and less subject to bias than other outcomes such as infection; and therefore will likely remain a core outcome in critical care nutrition studies, particularly large pragmatic trials.
Do we need larger trials to detect smaller treatment effect?
The lack of benefit of increased caloric intake appears to be a consistent finding across the different studies. A recent meta-analysis of six trials and 2,517 patients demonstrated no difference in the risk of hospital-acquired infections, hospital mortality, ICU length of stay or ventilator-free days between patients receiving intentional hypocaloric versus normocaloric nutritional goals (25). Thus, current data demonstrate that in general, restricted compared to full caloric intake during the acute phase of critical illness does not affect mortality. However, we believe that there is a need to better assess who may or may not benefit from nutritional interventions; and a need for further adequately powered trial in these target groups.
Should we use different endpoints than mortality? How about biomarkers?
We believe the response to both of these questions is ‘Yes’. In particular, the effect of nutritional support on functional outcomes and quality of life should be systematically studied. These patient-centered outcomes have been incorporated in several recent trials as secondary endpoints or in a subset of patients (Table 1). The role of an integrated intervention that includes nutrition and mobilization needs further study; and functional outcomes will be essential to measure the effect of such intervention. Another important endpoint in nutrition studies is kidney function. In post-hoc analysis of the PermiT trial, need for renal replacement was lower in the permissive underfeeding group. As pointed out by Drs. Casaer and Van den Berghe, this is consistent with the findings of the EPaNIC trial which demonstrated longer median duration of renal-replacement therapy in the early parental nutrition group (6,26). It is also consistent with the Nephroprotective trial which found a trend towards increased renal replacement in patients receiving amino acid therapy compared to standard therapy (27). Animal studies have also shown beneficial effects of short-term calorie restriction on renal and vascular ischemia-reperfusion injury (28,29). Therefore, renal function should be an a priori outcome in nutrition trials in critically ill patients. Incorporating biomarkers in nutrition studies is important to better understand the underlying pathophysiologic effects. The PermiT Trial demonstrated that increasing caloric intake did not affect parameters of protein metabolism, as reflected by prealbumin, transferrin and nitrogen balance (1). Further work is underway to examine the effects of caloric dose on inflammation and oxidative stress in patients enrolled in the PermiT trial. While biomarkers are important, they will not replace important patient-centered outcomes, but will provide additional information about mechanisms.
Are specific patient groups more likely to be nutrition-responsive?
As indicated by Drs. Casaer and Van den Berghe, the patients enrolled in the PermiT trial were those considered most likely to be affected by nutritional interventions (predominantly non-surgical, many suffering from sepsis at inclusion, with a median ICU stay of about 13 days). Yet, permissive underfeeding compared to standard feeding did not affect the outcomes of such patients. Drs. Casaer and Van den Berghe noted that patients with hyperglycemia (>9.2 mmol/L) at randomization may be a subgroup that may benefit from permissive underfeeding compared with standard feeding (relative risk, 0.83; 95% confidence interval, 0.63–1.1, P=0.19) (1). Further work is needed to identify subsets of patients who may benefit from feeding below energy targets.
How generalizable are the results of PermiT to normal weight or underweight patient populations? Are there specific groups more likely to be nutrition-responsive?
These important questions are relevant to most of the recent trials, given that the majority of patients had an average body mass index (BMI) of 25–30, including a trial of patients with refeeding syndrome (5) (Table 1). This is a reflection of the BMI in the general population (for example, the age-adjusted average BMI in the United States is 29) (30). There is a need for studies evaluating patients with different BMI groups, however as indicated by Drs. Casaer and Van den Berghe, studies have shown that the nutritional effect may not be differ among different BMI groups, as BMI may not be the best measure for underlying nutritional status. While alternative approaches to assess nutritional status such as the Nutrition Risk in Critically ill (NUTRIC) score have been proposed (31), their ability to discriminate nutrition-responsive patients needs further evaluation.
We fully agree that the next pressing question is the effect of protein intake on critically ill patients. Recent trials have used different strategies for protein intake. It remains unclear whether more protein is associated with better outcomes through preservation of muscle mass (32), or with worse outcomes through inhibition of autophagy as suggested by Drs. Casaer and Van den Berghe.
Recent trials such as PermiT have helped paved the path on our journey to better understanding of the effect of nutrition on the outcome of critically ill patients. This journey is far from over, and is certain to be an exciting one.
Acknowledgements
This work is supported by King Abdullah International Medical Research Center, Riyadh, Saudi Arabia.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Arabi YM, Aldawood AS, Haddad SH, et al. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N Engl J Med 2015;372:2398-408. [Crossref] [PubMed]
- Casaer MP, Van den Berghe G. Editorial on the original article entitled "Permissive underfeeding of standard enteral feeding in critically ill adults" published in the New England Journal of Medicine on June 18, 2015. Ann Transl Med 2015;3:226. [PubMed]
- 2012.
- Rice TW, Mogan S, Hays MA, et al. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med 2011;39:967-74. [Crossref] [PubMed]
- Doig GS, Simpson F, Heighes PT, et al. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med 2015;3:943-52. [Crossref] [PubMed]
- Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011;365:506-17. [Crossref] [PubMed]
- Doig GS, Simpson F, Sweetman EA, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA 2013;309:2130-8. [Crossref] [PubMed]
- Harvey SE, Parrott F, Harrison DA, et al. Trial of the route of early nutritional support in critically ill adults. N Engl J Med 2014;371:1673-84. [Crossref] [PubMed]
- Heidegger CP, Berger MM, Graf S, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet 2013;381:385-93. [Crossref] [PubMed]
- Needham DM, Dinglas VD, Bienvenu OJ, et al. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ 2013;346:f1532. [Crossref] [PubMed]
- Needham DM, Dinglas VD, Morris PE, et al. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med 2013;188:567-76. [Crossref] [PubMed]
- Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med 2001;29:2264-70. [Crossref] [PubMed]
- Arabi YM, Haddad SH, Tamim HM, et al. Near-target caloric intake in critically ill medical-surgical patients is associated with adverse outcomes. JPEN J Parenter Enteral Nutr 2010;34:280-8. [Crossref] [PubMed]
- Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: depends on how you slice the cake! Crit Care Med 2011;39:2619-26. [Crossref] [PubMed]
- Rubinson L, Diette GB, Song X, et al. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med 2004;32:350-7. [Crossref] [PubMed]
- Ibrahim EH, Mehringer L, Prentice D, et al. Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial. JPEN J Parenter Enteral Nutr 2002;26:174-81. [Crossref] [PubMed]
- Arabi YM, Tamim HM, Dhar GS, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 2011;93:569-77. [Crossref] [PubMed]
- Lin SJ, Kaeberlein M, Andalis AA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 2002;418:344-8. [Crossref] [PubMed]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 2003;78:361-9. [PubMed]
- Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004;305:390-2. [Crossref] [PubMed]
- Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 2006;295:1539-48. [Crossref] [PubMed]
- Dandona P, Mohanty P, Ghanim H, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 2001;86:355-62. [PubMed]
- Gredilla R, Sanz A, Lopez-Torres M, et al. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J 2001;15:1589-91. [PubMed]
- Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol 2008;295:H2348-55. [Crossref] [PubMed]
- Marik PE, Hooper MH. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: a systematic review and meta-analysis. Intensive Care Med 2016;42:316-23. [Crossref] [PubMed]
- Gunst J, Vanhorebeek I, Casaer MP, et al. Impact of early parenteral nutrition on metabolism and kidney injury. J Am Soc Nephrol 2013;24:995-1005. [Crossref] [PubMed]
- Doig GS, Simpson F, Bellomo R, et al. Intravenous amino acid therapy for kidney function in critically ill patients: a randomized controlled trial. Intensive Care Med 2015;41:1197-208. [Crossref] [PubMed]
- Mitchell JR, Verweij M, Brand K, et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 2010;9:40-53. [Crossref] [PubMed]
- Mauro CR, Tao M, Yu P, et al. Preoperative dietary restriction reduces intimal hyperplasia and protects from ischemia-reperfusion injury. J Vasc Surg 2016;63:500-509.e1. [Crossref] [PubMed]
- Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491-7. [Crossref] [PubMed]
- Heyland DK, Dhaliwal R, Jiang X, et al. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 2011;15:R268. [Crossref] [PubMed]
- Singer P, Hiesmayr M, Biolo G, et al. Pragmatic approach to nutrition in the ICU: expert opinion regarding which calorie protein target. Clin Nutr 2014;33:246-51. [Crossref] [PubMed]


