
Performance evaluation of the BC-720 auto hematology analyzer and establishment of the reference intervals of erythrocyte sedimentation rate in healthy adults
Introduction
Erythrocyte sedimentation rate (ESR) is the rate at which erythrocytes sediment under certain conditions and is related to the morphology, surface charge, and components of erythrocytes, such as immunoglobulin and fibrinogen. The specificity of the ESR in the diagnosis of diseases is not high, but it can provide certain information about the quiescent and active stages of diseases, the stability and recurrence of diseases, and the differentiation of benign and malignant tumors. An increase in ESR can suggest rheumatoid arthritis, infection, tumors, lupus erythematosus, multiple myeloma, and other diseases, making it a commonly used nonspecific test item (1,2). The Westergren method, as a traditional ESR detection method, is recommended by the International Council for Standardization in Hematology (ICSH) as the standard method for ESR detection (3). Even so, it is hampered by technical factors such as temperature, sampling time, direction of the erythrocyte sedimentation tube, and vibration (4). It is time-consuming to perform in laboratories with a heavy workload and is not easy to scale up. Therefore, semiautomated and fully automated ESR analyzers were introduced to the market approximately 40 years ago, heralding a new era for ESR detection. Semiautomated and fully automated analyzers have greatly shortened the analysis time of ESR, and they can use ethylenediaminetetraacetic acid (EDTA)-anticoagulated blood for detection, which improves their sample stability and ensures operator safety by reducing the risk of biological exposure (5).
The Mindray BC-720 automated hematology analyzer (Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) combines blood cell analysis with ESR detection and uses the sheath flow impedance method, laser light scattering method, and flow cytometry combined with fluorescence staining for cell classification and counting. At the same time, it uses the photometric method to measure the degree of red blood cell aggregation within a specified time to calculate the ESR. A change in the aggregation state of erythrocytes leads to changes in the scattering effect of the erythrocytes on the measurement beam, the specific manifestation of which is that the light transmittance increases with greater aggregation. The degree of aggregation of erythrocytes is obtained by measuring the light transmittance of a whole-blood sample over time, and this is used to calculate the ESR. This blood cell analyzer can obtain ESR results while obtaining routine blood results, so it can be used in most clinical departments.
In this study, we evaluated the repeatability and carryover rate (CR) of the Mindray BC-720 automated hematology analyzer, and compared this method with the Westergren method. Samples from healthy populations of different genders and ages in Nanjing, Suzhou, and Guangzhou were collected to construct the BC-720 ESR reference interval. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3486/rc).
Methods
Performance evaluation, data verification, and reference range verification of BC-720 ESR were performed according to ICSH [2017] (6), the Clinical and Laboratory Standards Institute (CLSI) (H02-A5) (7), and H26-A2 (8). This study involved in vitro diagnostic clinical evaluation. Leftover samples with reports already issued by the hospital were used to obtain the corresponding research data after detection by our instruments. The patient’s privacy and interests were not involved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Zhongda Hospital Southeast University (SUZH) (No. 2021ZDSYLL335-P01). All participating hospitals were informed and agreed the study. Due to the study’s retrospective nature, the requirement to obtain signed informed consent from the patients was waived. Data are not available due to ethical restrictions.
Sample collection
Methodological validation
In this study, outpatients or inpatients of SUZH underwent daily ESR tests, and routine blood samples from the same day were collected for BC-720 and Westergren tests. Blood samples from healthy people were selected during physical examination for the establishment of the reference interval. Samples taken from women during menstruation or pregnancy and from patients who had recently undergone recent surgery or experienced infection were excluded. The volume of each sample was not less than 2 mL. Following the CLSI H04-A6 (8,9) standard, samples with hemolysis and coagulation were not included in the assessment. The samples were kept at room temperature (18–26 ℃), and the test was completed within 4 hours after collection.
Reference interval survey
A total of 3,233 samples were collected from healthy individuals of different age groups during physical examinations at the SUZH, The First Affiliated Hospital of Soochow University (SUH), and the Sun Yat-sen Memorial Hospital (SYU). Among the volunteers were 1,442 males (21–86 years old) and 1,791 females (21–88 years old). The participants were grouped by gender and then grouped by age. All specimens were tested within 4 hours after blood collection.
ESR test methods
ESR test by the Westergren method (reference method)
The EDTA-K2-anticoagulated whole blood was thoroughly mixed with 109 mmol/L sodium citrate solution at a ratio of 4:1. The samples were always tested within 4 hours. After filling with the mixed sample, the ESR tube (Biosigma, Venice, Italy) was placed in a special ESR rack. The ESR tube was kept upright (within 1°) and allowed stand for 60 minutes at a constant temperature (18–25 ℃). During the measurement period, the indoor temperature change was less than 1 ℃, and touching, moving, and tabletop vibrations were avoided. After 60 minutes (±1 min), the horizontal visual reading was recorded. The unit was recorded in mm/h, and the minimum reading value was 1 mm/h.
Easy-W ESR method (BC-720 detection method)
To detect the ESR by the BC-720 auto hematology analyzer, complete blood count (CBC) + differential counts (DIFF) + reticulocyte count (RET) (CDR) + ESR mode was used. The volume of whole blood was 160 µL, and the test time was approximately 2 minutes. Both 5-item routine blood results and ESR results can be reported simultaneously.
ESR test by the modified Westergren method (LBY-XC40B)
We mixed 2 mL of sodium citrate-anticoagulated blood with a ratio of sodium citrate to venous blood of 1:4 by inverting 8–10 times, and 1.5 mL of blood was drawn using a special syringe and injected into the disposable ESR tube (below the scale line). The disposable ESR tube was gently inserted into the test hole of the auto dynamic ESR tester (Beijing Precil Instrument Co., Ltd., Beijing, China) for auto scanning. The ESR was read after 30 minutes.
Basic performance verification of the BC-720 ESR
Carryover
Samples with ESR ≥80 mm/h were selected as high-target-value (HTV) samples, and samples with ESR ≤5 mm/h were selected as low-target-value (LTV) samples, with a total of 9 groups. Then, 3 consecutive HTV tests and 3 consecutive LTV tests on the selected samples were performed in the BC-720 automated hematology analyzer in CDR + ESR mode, yielding a total of 6 results, named H1, H2, H3, L1, L2, and L3. The CR was calculated according to the following formula: CR% = (L1 − L3)/(H3 − L3) × 100%.
Repeatability
A total of 22 ESR samples, covering the whole reportable interval (0–140 mm/h), were selected. After each sample had been fully mixed, the measurement was repeated 10 times in the BC-720 in CDR + ESR mode. The mean value, standard deviation (SD), and coefficient of variation (CV) of each group were calculated. Efforts were made to complete the 10 tests of each sample within 30 minutes.
Methodological comparison
A total of 212 venous blood samples were collected from patients who had ESR tests in the outpatient and inpatient departments of SUZH and were tested by the BC-720 and the standard Westergren method. In addition, the sodium citrate-anticoagulated ESR samples of the hospital were measured again using the LBY-XC40B. Passing-Bablok regression analysis was used for analysis, and r values were calculated.
Statistical analysis
The software SPSS 21.0 (IBM Corp., Armonk, NY, USA) was used for data processing. Methodological comparison was performed using linear regression analysis. The Pauta criterion was used to analyze the outliers, and the outliers were excluded. The Kolmogorov-Smirnov test was performed to test variables for normality, in which P>0.05 indicated that the data were normally distributed. The paired samples were tested using the t-test for means; the biological reference interval with a normal distribution was calculated using ; and percentile analysis was used for nonnormal distributions. The Mann-Whitney U test was used to determine whether there were differences between genders. A P value <0.05 was considered statistically significant.
Results
Carryover
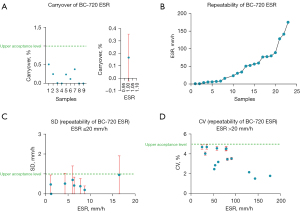
Figure 1A shows the carryover results of the test samples. The carryover of the CR was less than 1%, as shown in the left panel. The right panel shows the mean carryover of the samples.
Repeatability
The results of sample repeatability are shown in Figure 1. Figure 1B shows the mean repeatability of the 22 blood samples with a wide range of ESR values detected by the BC-720 auto hematology analyzer. Among them, those with ESR ≤20 mm/h and SD <1 mm/h are shown in Figure 1C. The CV of the samples with ESR >20 mm/h was <5%, as shown in Figure 1D. The above results all met the manufacturer’s claims.
Methodological comparison
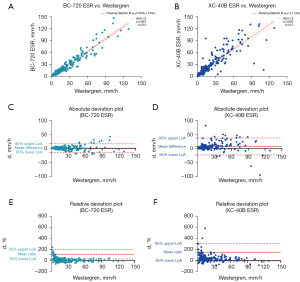
The venous blood samples of 212 outpatients and inpatients were randomly selected for ESR detection using the BC-720 auto hematology analyzer and the manual Westergren method. The results are shown in Figure 2A. On the X-axis is the ESR result of Westergren method, and on the Y-axis is the ESR result measured by the BC-720. The Passing-Bablok regression analysis showed that the ESR detected by the BC-720 had a good correlation with the ESR result of the Westergren method (y = 0.359 + 1.016x, r=0.957). In Figure 2B, the X-axis is the ESR result of the Westergren method, and the Y-axis is the ESR result of the LBY-XC40B test. The Passing-Bablok regression analysis yielded y = 1 + 1.25x and r=0.856. The correlation between the 2 was lower than the correlation between the BC-720 and the Westergren method. Figure 2C,2D show the absolute deviations of the BC-720 and the Westergren method and the absolute deviations of the LBY-XC40B and the Westergren method, respectively. It can be seen that the absolute deviation between the BC-720 and the Westergren method was smaller. Figure 2E,2F show the relative deviations between the BC-720 and the Westergren method and the relative deviations between the LBY-XC40B and the Westergren method, respectively. Again, the relative deviation between the BC-720 and the Westergren method was smaller. In summary, the BC-720 hematology analyzer had a good correlation with the Westergren method, and the deviation was small.
Reference intervals
ESR reference interval in east China
A total of 2,349 individuals who underwent physical examination were enrolled from SUZH and SUH, including 1,054 males and 1,295 females. Their ESR results were tested for outliers using the Pauta criterion, and 43 were found and excluded. The ESR results of the remaining 2,306 healthy people are shown in Table 1, including 1,029 males and 1,277 females.
Table 1
| Age (years) | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P50 | P2.5 | P97.5 | N | P50 | P2.5 | P97.5 | ||
| 21–30 | 114 | 4.06 | 1.05 | 9.62 | 310 | 6.06 | 2.11 | 15.48 | |
| 31–40 | 308 | 4.00 | 1.13 | 10.07 | 383 | 6.52 | 3.41 | 18.80 | |
| 41–50 | 242 | 3.95 | 1.01 | 8.81 | 254 | 6.89 | 2.55 | 15.86 | |
| 51–60 | 223 | 4.44 | 1.69 | 10.57 | 206 | 7.27 | 1.81 | 21.43 | |
| >60 | 142 | 4.87 | 1.19 | 12.38 | 124 | 8.28 | 4.47 | 21.27 | |
ESR, erythrocyte sedimentation rate.
ESR reference interval in south China
A total of 965 participants who underwent physical examination were collected from SYU, including 406 males and 559 females. Their ESR results were tested for outliers using the Pauta criterion, and 50 were found and excluded. The ESR results of the remaining 915 healthy volunteers are shown in Table 2, including 385 males and 530 females.
Table 2
| Age (years) | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P50 | P2.5 | P97.5 | N | P50 | P2.5 | P97.5 | ||
| 21–30 | 101 | 4.19 | 0.63 | 10.90 | 145 | 8.60 | 4.49 | 27.40 | |
| 31–40 | 127 | 4.49 | 0.92 | 12.44 | 165 | 7.95 | 4.25 | 23.77 | |
| 41–50 | 65 | 4.23 | 0.88 | 15.31 | 79 | 10.36 | 4.91 | 29.72 | |
| 51–60 | 64 | 4.53 | 0.58 | 16.64 | 95 | 13.55 | 4.62 | 30.25 | |
| >60 | 28 | 7.20 | 1.98 | 19.92 | 46 | 12.46 | 3.81 | 29.36 | |
ESR, erythrocyte sedimentation rate.
Kolmogorov-Smirnov normality test on the overall data
A total of 3,315 participants who underwent physical examination were enrolled from the 3 hospitals. The outlier test was performed on the ESR results using the Pauta criterion, and 82 outliers were found and eliminated. The ESR results for the remaining 3,233 healthy people are shown in Table 3. Among them, 1,442 were males and 1,791 were females, with an age range of 21 to 88 years. The frequency distribution of ESR results is shown in Figure 3A, and the results of the normality test is shown Figure 3B.
Table 3
| Hospital | Male | Female |
|---|---|---|
| SUZH | 589 | 604 |
| SUH | 466 | 592 |
| SYU | 406 | 658 |
| Total samples | 1,461 | 1,854 |
| Final samples | 1,442 | 1,791 |
SUZH, Zhongda Hospital Southeast University; SUH, The First Affiliated Hospital of Soochow University; SYU, Sun Yat-sen Memorial Hospital.
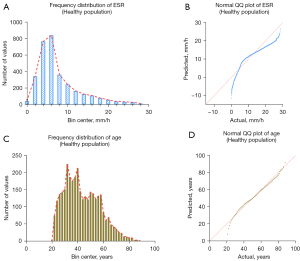
The frequency distribution and normality test of age are shown in Figure 3C,3D. It can be seen that the data were all non-normally distributed, so P2.5–P97.5 was used as the reference interval of ESR (since the low value of ESR has no obvious clinical value, the P0–P97.5 confidence interval was chosen).
Mann-Whitney U test
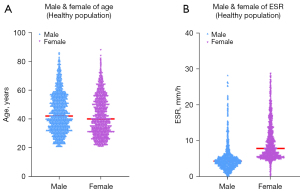
Due to the influence of gender on ESR values, participants were grouped by gender, and the Mann-Whitney U test was used to compare them. Figure 4A shows the results of the Mann-Whitney U test for the age distributions of males and females (Z=−5.038; P<0.01). Figure 4B shows the distribution of ESR in healthy males and females. There was a significant difference in the ESR results between males and females (Z=−33.078; P<0.01). Therefore, it was necessary to establish reference intervals for males and females separately.
Kruskal-Wallis H test
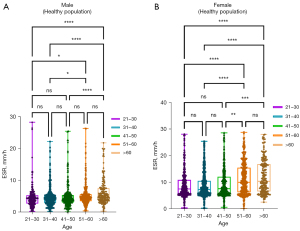
The data were divided into 5 age groups of 10-year intervals. The results of the ESR percentile estimation are shown in Table 4. The Kruskal-Wallis H test was used to compare ESR between age groups (Figure 5A). After pairwise comparison, no significant differences were found between the age groups of the healthy male population (P>0.05). As shown in Figure 5B, after pairwise comparison, there was only a difference between the 41–50-year-old group and the 51–60-year-old group of females (P<0.01). Therefore, the females were divided into two groups for the Wilcoxon test using 50 years old as the cutoff value, and their ESRs were significantly different (P<0.01).
Table 4
| Age (years) | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P50 | P2.5 | P97.5 | N | P50 | P2.5 | P97.5 | ||
| 21–30 | 219 | 4.14 | 0.68 | 12.67 | 402 | 7.17 | 3.40 | 22.92 | |
| 31–40 | 440 | 4.19 | 1.00 | 12.62 | 546 | 7.20 | 3.83 | 20.82 | |
| 41–50 | 317 | 4.04 | 1.00 | 18.53 | 344 | 7.75 | 3.31 | 24.19 | |
| 51–60 | 292 | 4.46 | 1.28 | 15.00 | 319 | 9.94 | 3.10 | 24.41 | |
| >60 | 174 | 5.02 | 1.37 | 18.93 | 180 | 10.28 | 4.23 | 26.27 | |
ESR, erythrocyte sedimentation rate.
The percentile method was used to estimate the ESR reference intervals, and the results are shown in Table 3. Due to the small difference in the ESR reference intervals between the female age groups (only 2 mm/h), after comprehensively considering the real-world application situation, the reference intervals were statistically analyzed according to gender only. The results of this analysis are shown in Table 5.
Table 5
| Gender | Age | N | ESR (mm/h) | |
|---|---|---|---|---|
| P50 | P2.5–P97.5 | |||
| Male | >20 | 1,442 | 4.28 | 1.00–15.28 |
| Female | 21–50 | 1,292 | 7.35 | 3.49–23.09 |
| >50 | 499 | 10.00 | 3.70–25.18 | |
| Final ESR reference range | Male ≤15 mm/h; female ≤24 mm/h | |||
ESR, erythrocyte sedimentation rate.
Discussion
The ESR is the rate at which erythrocytes sediment under certain conditions. The ESR values of healthy individuals usually fluctuate within a relatively narrow range. However, the ESR is pathologically increased in patients with various inflammatory diseases, tuberculosis, rheumatic fever, tissue damage or necrosis, malignant tumors, rheumatoid arthritis, systemic lupus erythematosus, chronic nephritis, liver cirrhosis, multiple myeloma, giant cell arteritis, anemia, and even coronavirus disease of 2019 (COVID-19) (1,10-12). The ICSH recommends the Westergren method as the reference method for ESR detection, but it is time-consuming, requires a large amount of blood, is not easy to perform on a large scale, and has certain safety risks (13). The BC-720 automated hematology analyzer uses the sheath flow impedance method, the laser light scattering method, and flow cytometry combined with fluorescence staining for cell classification and counting. In addition, the photometric method is used to measure the degree of erythrocyte aggregation within a specified time to calculate the ESR. In addition to routine blood results, the BC-720 outputs the ESR. It easy to operate, reduces the risk of additional biological exposure, can speed up testing, and can benefit laboratory departments or clinical laboratories with large workloads.
The BC-720 automated hematology analyzer is the latest blood cell analyzer launched by Mindray. It can perform ESR detection while performing routine blood tests. The Mindray Easy-W method can quickly and accurately determine the ESR. The Easy-W ESR detection technology first simulates blood flow in a blood vessel by driving the blood sample to flow in a high-speed laminar flow within the detection tube. The shear force of the laminar flow causes the erythrocyte mass to depolymerize to a monodisperse state and stay in that state. By accurately controlling the depolymerization flow rate and tubing size, the maximum shear rate in the tubing detected by Easy-W is approximately 1,050 s−1, which is higher than the maximum shear rate in normal human blood vessels, thereby ensuring complete depolymerization of erythrocytes. At a high speed of flow, the erythrocytes depolymerize under the action of shearing force; at the same time, they are arranged in a consistent orientation along the shearing direction to produce tensile deformation. After the shearing effect disappears, the erythrocytes first return to a random orientation and to their normal biconcave disc shape, and then they begin to aggregate (14). The aggregation rate peaks early and then gradually slows down as the degree of aggregation increases. Therefore, the accurate measurement of the aggregation rate early on in aggregation is key. At the same time, Easy-W ESR technology adopts near-infrared photometry to measure the aggregation process of erythrocytes in real time. Not only is it sensitive to erythrocyte aggregation, but it also ensures that the measurement of the degree and speed of erythrocyte aggregation is not affected by factors such as blood oxygen saturation (15). In addition, the Easy-W ESR measurement assembly maintains a constant temperature of 37 ℃ to simulate the temperature of the human body, effectively avoiding the impact of environmental temperature changes on the ESR results, ensuring the stability of the measurement results, and improving its correlation with the gold-standard Westergren method. At present, most medical institutions in China follow the reference interval of the Westergren method, and there are no reports on the ESR reference interval of the BC-720 hematology analyzer. We thought it was necessary to establish an appropriate reference interval for the BC-720 hematology analyzer.
This study evaluated the ESR detection performance of the BC-720 automated hematology analyzer from the aspects of repeatability, CR, and comparability with the Westergren method. The repeatability and CR of the ESR results of the BC-720 hematology analyzer met the manufacturer’s claims. In terms of accuracy, the ESR results measured by the BC-720 correlated well with the ESR results measured by the Westergren method, with low absolute deviation and relative deviation. In summary, the BC-720 automated hematology analyzer has good repeatability, a low CR, and high accuracy compared with the Westergren method, so it can be used for sample detection in clinical laboratories.
To determine the biological reference interval of the ESR, we first analyzed the ESR in east China and south China. We enrolled 2,306 medical examination samples from SUZH and SUH in east China and 915 medical examination samples from SUY in south China. The statistical analysis showed that the ESR results of males in south China and east China were different at different ages, and the ESR values in south China were all higher than those in east China. The ESR results were significantly higher among women in south China than those in east China (P<0.01). This may be due to the differences in altitude and geographical environment between south China and east China. To preliminarily establish the ESR reference intervals of the BC-720 hematology analyzer, the physical examination samples from the 3 hospitals were combined for analysis.
In the total of 3,292 physical examination samples of healthy adults, outliers were removed according to the Pauta criterion, totaling 59. Finally, a total of 3,233 healthy physical examination samples were included in the analysis, including 1,442 males and 1,791 females. The Kolmogorov-Smirnov normality test suggested that the ESR values of the healthy population showed a skewed distribution. The percentile method was used to analyze the reference interval (P2.5–P97.5). Subsequently, the Mann-Whitney U test was used for analysis, which found that the differences in ESR results between males and females were statistically significant (Figure 4). This necessitated establishing the reference intervals for males and females separately. Afterward, the Kruskal-Wallis H test was used to examine whether there were differences between age groups in the males and females. The analysis showed that there was no significant difference in the ESR distribution between age groups in the healthy male population (P>0.05), while statistically significant differences existed only between the 41–50-year-old group and the 51–60-year-old group of females. Because the results of the two groups were not greatly different, grouping according to this age-group difference would not help with real-world clinical application and would increase the workload of clinicians, so no separate grouping was performed for the reference interval setup. The final reference interval was determined to be 0–15 mm/h for males and 0–24 mm/h for females. Using these ranges may result in the occurrence of false-negative or false-positive results in a small number of samples, so combining the specific values and other laboratory test results can reduce the impact of such results on clinical diagnosis.
The ESR reference intervals of the healthy population in this study were different from those of the Westergren method (male: 0–15 mm/h; female: 0–20 mm/h), which may be caused by differences in populations from different regions. The ESR has also been associated with lifestyle factors, such as physical activity, smoking, and drinking (16,17). Changes in people’s living habits may also lead to changes in ESR values. The reference intervals used by the Westergren method was developed many years ago, and key population characteristics may have changed since then. The results of this study showed that gender had a great impact on ESR values, the ESR value of women being higher than that of men, which is also consistent with previous reports (18). China has a vast territory, many ethnic groups, and different geographical environments and living habits. Therefore, it would be difficult to complete a large-scale national survey. According to existing conditions, this study investigated the ESR reference intervals of the healthy population in south China and east China. Although the sample coverage of this survey was not extensive, our findings provide preliminary ESR reference intervals for south China and east China. Although there was a small gap with the results of the Westergren method, these results still have great clinical reference value. Clinical laboratories should establish their own reference intervals according to their actual situation to ensure their clinical applicability.
In this study, the basic performance of the BC-720 instrument was first verified, and then the comparability of the Easy-W method with the Westergren method was evaluated. Finally, the reference interval of the healthy population was investigated. The final ESR reference intervals of the BC-720 hematology analyzer are 0–15 mm/h for males and 0–24 mm/h for females. In summary, the BC-720 hematology analyzer has good repeatability and a low carryover and has a good correlation with the Westergren method. Each laboratory can establish its own reference interval according to its actual situation to provide a reliable basis for clinical practice and to maximize the value of the BC-720 in ESR clinical detection.
Acknowledgments
Funding: This study was funded by the National Natural Science Foundation of China (NSFC) (No. 81872618).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3486/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3486/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3486/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Zhongda Hospital Southeast University (SUZH) (No. 2021ZDSYLL335-P01). All participating hospitals were informed and agreed the study. Due to the study’s retrospective nature, the requirement to obtain signed informed consent from the patients was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Plebani M. Erythrocyte sedimentation rate: innovative techniques for an obsolete test? Clin Chem Lab Med 2003;41:115-6. [Crossref] [PubMed]
- Reinhart WH. Erythrocyte sedimentation rate--more than an old fashion? Ther Umsch 2006;63:108-12. [Crossref] [PubMed]
- Reference method for the erythrocyte sedimentation rate (ESR) test on human blood. Br J Haematol 1973;24:671-3. [Crossref] [PubMed]
- Ramsay ES, Lerman MA. How to use the erythrocyte sedimentation rate in paediatrics. Arch Dis Child Educ Pract Ed 2015;100:30-6. [Crossref] [PubMed]
- Pieri M, Pignalosa S, Perrone MA, et al. Evaluation of the Diesse Cube 30 touch erythrocyte sedimentation method in comparison with Alifax test 1 and the manual Westergren gold standard method. Scand J Clin Lab Invest 2021;81:181-6. [Crossref] [PubMed]
- Kratz A, Plebani M, Peng M, et al. ICSH recommendations for modified and alternate methods measuring the erythrocyte sedimentation rate. Int J Lab Hematol 2017;39:448-57. [Crossref] [PubMed]
- Clinical and Laboratory Standards Institute. Procedures for the Erythrocyte Sedimentation Rate Test; Approved Standard—Fifth Edition. Wayne: Clinical and Laboratory Standards Institute, 2011:H02-A5.
- Clinical and Laboratory Standards Institute. Validation, Verification, and Quality Assurance of Automated Hematology Analyzers; Approved Standard—Second Edition. Wayne: Clinical and Laboratory Standards Institute, 2010:H26-A2.
- Clinical and Laboratory Standards Institute. Procedures and Devices for the Collection of Diagnostic Capillary Blood Specimens; Approved Standard—Sixth Edition. Wayne: Clinical and Laboratory Standards Institute, 2008:GP42-A6.
- Qiu J, Lu C, Zhang L, et al. Osteoporosis in patients with rheumatoid arthritis is associated with serum immune regulatory cellular factors. Clin Rheumatol 2022; Epub ahead of print. [Crossref] [PubMed]
- Watanabe R, Berry GJ, Liang DH, et al. Pathogenesis of Giant Cell Arteritis and Takayasu Arteritis-Similarities and Differences. Curr Rheumatol Rep 2020;22:68. [Crossref] [PubMed]
- Qin R, He L, Yang Z, et al. Identification of Parameters Representative of Immune Dysfunction in Patients with Severe and Fatal COVID-19 Infection: a Systematic Review and Meta-analysis. Clin Rev Allergy Immunol 2022; Epub ahead of print. [Crossref] [PubMed]
- Paulus HE, Brahn E. Is erythrocyte sedimentation rate the preferable measure of the acute phase response in rheumatoid arthritis? J Rheumatol 2004;31:838-40. [PubMed]
- Schmid-Schönbein H, Kline KA, Heinich L, et al. Microrheology and light transmission of blood. III. The velocity of red cell aggregate formation. Pflugers Arch 1975;354:299-317. [Crossref] [PubMed]
- Uyuklu M, Canpolat M, Meiselman HJ, et al. Wavelength selection in measuring red blood cell aggregation based on light transmittance. J Biomed Opt 2011;16:117006. [Crossref] [PubMed]
- Alende-Castro V, Alonso-Sampedro M, Vazquez-Temprano N, et al. Factors influencing erythrocyte sedimentation rate in adults: New evidence for an old test. Medicine (Baltimore) 2019;98:e16816. [Crossref] [PubMed]
- Siemons L, Ten Klooster PM, Vonkeman HE, et al. How age and sex affect the erythrocyte sedimentation rate and C-reactive protein in early rheumatoid arthritis. BMC Musculoskelet Disord 2014;15:368. [Crossref] [PubMed]
- Vennapusa B, De La Cruz L, Shah H, et al. Erythrocyte sedimentation rate (ESR) measured by the Streck ESR-Auto Plus is higher than with the Sediplast Westergren method: a validation study. Am J Clin Pathol 2011;135:386-90. [Crossref] [PubMed]
(English Language Editor: J. Jones)


