
A comprehensive framework of the right posterior section for tailored anatomical liver resection based on three-dimensional simulation system
Introduction
Current liver surgery has reached a high level of complexity that requires not only expert skills but also well-integrated interdisciplinary schemes to achieve optimal outcomes for patients. Anatomical liver resection (ALR) defined as the complete removal of the territory supplied by the respective Glissonean pedicle represents the paradigm of surgical care that may decrease recurrence risk and improve long-term survival for patients with benign or malignant hepatobiliary disease (1-3). Among various types of hepatectomy, anatomical right posterior sectionectomy (ARPS) is deemed as a highly specialized and risky procedure in both laparoscopic and open liver resections owing to the difficulty in the exposure of the deeply located lesion, isolation of the targeted Glissonean pedicle, and determination of the accurate cutting plane during parenchymal transection (4,5).
Hepatic parenchymal transection guided by the demarcation line after clamping or staining the portal branches and the signpost venous branches running along the intersegmental plane is a well-established method for ALR (1,2). Recently, indocyanine green (ICG) fluorescence navigation has been adapted for ALRs with persistent, three-dimensional (3D) parenchymal staining of the target territory (6). Yet the dissociation between portal territory-oriented demarcation and the venous trunk-oriented plane is remarkable, especially in the right lobe (7). Detailed knowledge of vascular territories and the intersegmental plane remains the prerequisite for conducting a precise ALR as well as minimizing complications considering anatomical complexity and vascular variability of the right posterior section (8). Fortunately, modern computer-assisted radiology has made major contributions in visualizing the portal pedicles and the hepatic veins as well as computing the number and the volumes of vascular territories that allow safe and precise liver resections (9,10). Several studies have reported the biliary vascular anatomy of the right posterior section, most focusing on the hepatic vein drainage areas or portal venous variations (11-13). However, little is known about the portal vein variations related to the aberrant right intersegmental plane between the right anterior lobe and the right posterior lobe. We aimed to reveal the relationship between the aberrant demarcation of the right posterior portal territory and the portal venous variation, therefore develop a comprehensive framework of the right posterior section for ARPS or other alternative safe ALR based on a 3D hepatectomy simulation system, and validate its clinical usefulness in a rigorous prospective manner. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1105/rc).
Methods
Patients
Healthy participants who underwent triple-phase contrast-enhanced computed tomography (CECT) of the upper abdomen between July 2018 and June 2020 at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China) were retrospectively evaluated to propose a unified framework for ARPS. Exclusion criteria were as follows: (I) presence of large liver lesion or liver cirrhosis; (II) history of hepatobiliary surgery; (III) poor image quality; (IV) slice thickness >1.5 mm. On the other hand, we prospectively applied the proposed framework to the practice of ARPS for hepatocellular carcinoma (HCC) between July 2020 and June 2021. The flowchart of the study design is available in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (approval number: 2020-SR-444) and written informed consent was obtained from all patients.

CECT examination
Triple-phase (arterial-dominant, portal-dominant, and delayed phases) CECT scans were performed using a 64-multidetector or 128-multidetector CT scanner (SOMATOM Definition or SOMATOM Definition AS+, Siemens Medical Solutions, Forchheim, Germany) with the following parameters: 120 kV; 180 mAs; 0.5s rotation time; 32×1.2 mm collimation; 350×350 mm field-of-view; a 512×512 matrix; a slice thickness of 0.75–1.5 mm; a reconstruction interval of 0.75–1.5 mm. After intravenous administration of non-ionic iodinated contrast (Ultravist; 300 mgI/mL, Bayer Schering Pharma AG, Berlin, Germany) with a dose of 1.5 mL/kg and an injection rate of 3.0–4.0 mL/s, arterial- and venous-phase scans were acquired at 15 and 50 s after enhancement of the descending aorta to 100 HU, respectively. Delayed-phase scans started at a delay of 180 s after the acquisition of venous-phase scans.
3D simulation and framework
CECT data were saved in Digital Imaging and Communications in Medicine (DICOM) format and then transferred to Hisense computer-assisted surgery system (Hisense, Qingdao, China). 3D image processing was in a semi-automatic manner and consisted of the following steps: (I) automatic generation of the original 3D model based on uploaded CECT data; (II) manual modification of the liver contour as well as the portal and hepatic venous system by successive review of venous-phase images; (III) automatic integration of modified components to create the final 3D model; (IV) tailored calculation and visualization of the vascular perfusion or drainage territory based on volume-rendered hepatic vessels; (V) virtual ARPS simulation. All procedures were performed by a junior hepatobiliary surgeon (FZ with 2 years of experience in hepatobiliary surgery and imaging) under the supervision of a senior hepatobiliary surgeon (GJ with 8 years of experience in hepatobiliary surgery and imaging).
The integrated 3D images were viewed at various angles on the simulation workstation to examine the ramification pattern of the right posterior portal vein as well as the right hepatic venous system variation. The spatial relationship between the simulated transection plane determined by the right posterior portal territory (RPPT) and the course of the right hepatic vein (RHV) trunk was recorded to develop the framework. Note that the RPP refers to the RPP trunk or the bifurcation of segment 6 and 7 portal pedicles if there is no RPP trunk (13).
Statistical analysis
Continuous variables were presented as mean ± SD or median (Q1–Q3), where appropriate. Categorical variables were reported as frequencies with percentages unless otherwise indicated. Statistical analyses were performed with Student’s t-test and Man-Whitney U test, where appropriate. Aberrant volume proportion was defined as the proportion of the aberrant liver volume to the total liver volume. Statistical analysis was performed using SPSS statistical software (version 26.0, SPSS Inc., Chicago, IL, USA) and a two-sided P<0.05 was considered statistically significant.
Results
A total of 336 eligible participants who met the study criteria were enrolled for the proposal of the conceptual framework of the right posterior section. There were 167 men and 169 women with a median age of 59 (48–67) years. Among the 336 cases, 281 (83.6%) showed a typical Couinaud scheme, in which the RPP trunk divides into third-order branches for the right posterior section and the RHV drains the right posterior section, and 55 (16.4%) had no RPP trunk.
Morphological framework of the right posterior section
According to the dissociation between the intersegmental border determined by the RPPT and the course of the RHV trunk, the morphological framework of the right posterior section was summarized into four types (Table 1 and Figure 2). The normal (type I), caudal-redundant (type II), cranial-deficient (type III), and combined (type IV) types accounted for 43.4% (146 of 336), 25.3% (85 of 336), 18.5% (62 of 336) and 12.8% (43 of 336) of the total, respectively.
Table 1
| Type | Description | Quantity (percentages) |
|---|---|---|
| Type I: normal type | The intersegmental border determined by the RPPT coincides with the course of the RHV trunk | 146 (43.4) |
| Type II: caudal-redundant type | The intersegmental border determined by the RPPT protrudes caudal-laterally against the course of the RHV trunk | 85 (25.3) |
| Type III: cranial-deficient type | The intersegmental border determined by the RPPT sinks cranial-laterally against the course of the RHV trunk | 62 (18.5) |
| Type IV: combined type | The intersegmental border determined by the RPPT protrudes caudal-laterally and sinks cranial-laterally against the course of the RHV trunk | 43 (12.8) |
RPPT, right posterior portal territory; RHV, right hepatic vein.

In type II (caudal-redundant type), the caudal part of the simulated transection plane determined by the RPPT was dissociated with the course of the RHV trunk, tilting toward the ventral direction, while the cranial part almost coincided with the course of the RHV trunk. Two subtypes of portal venous variation were observed (Figure 3A,3B): (I) type IIa (59 of 85, 69.4%): the segment 6 portal branch (P6) running across the ventral side of RHV, defined as Ventral-P6 by Yamamoto et al. (14); (II) type IIb (26 of 85, 30.6%): a distal portal branch of P6 running across the ventral side of RHV. In type IIa configuration, single Ventral-P6 that originated near the hepatic hilum accounted for 94.9% (56 of 59). Two aberrant Ventral-P6 branches that one originated near the hepatic hilum and the other located away from the hepatic hilum, accounted for 5.1% (3 of 59). Unfortunately, the deep-located variable portal branches, either the second Ventral-P6 or the distal branch of P6, were less likely and risky to be preserved. The mean aberrant liver volume of type IIa was 88.3±44.3 mL, larger than that of type IIb (P<0.001), which was 58.4±25.1 mL. The Student’s t-test revealed that the mean aberrant volume proportion in type IIa was significantly greater than that in type IIb (P<0.001), which were 7.0%±3.5% and 4.4%±1.8%, respectively.
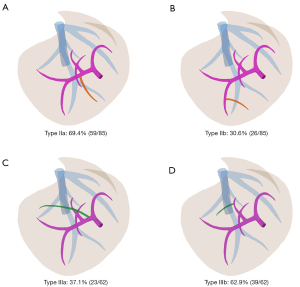
In type III (cranial-deficient type), the cranial part of the simulated transection plane determined by the RPPT was dissociated with the course of the RHV trunk, tilting toward the dorsal direction, while the caudal part coincided with the course of the RHV trunk. Two subtypes of portal venous variation were observed (Figure 3C,3D): (I) type IIIa (23 of 62, 37.1%): a segment 7 portal pedicle originating from the trunk of the right portal vein(RP), defined as RP-7; (II) type IIIb (39 of 62, 62.9%): the dorsal portal branch of segment 8, defined as P8c by Takayasu et al. (15), crossed over to the RHV without type IIIa variation. Note that 17.4% (4 of 23) of type IIIa cases were accompanied by type IIIb variation. Compared to the deep-located P8c, the RP-7 pedicle is easier to be controlled near the hepatic hilum. The median aberrant volume of type IIIa was 126.45 (104.43–173.25) mL, larger than that of type IIIb (P<0.001), which was 53.7 (37.3–87.9) mL. Man-Whitney U test revealed that the median aberrant volume proportion in type IIIa was significantly greater than that in type IIIb (P<0.001), which were 10.9% (8.5–13.3%) and 4.0% (3.0–6.1%), respectively.
Type IV (combined type) represented a combination of type II and type III, accounting for 12.8%. It was further classified into four subtypes (Figure 4): (I) type IVa (7.0%, 3 of 43): type IIa + IIIa; (II) type IVb (34.9%, 15 of 43): type IIa + IIIb; (III) type IVc (16.3%, 7 of 43): type IIb + IIIa; (IV) type IVd (41.8%, 18 of 43): type IIb + IIIb.

Tailored surgical strategies according to the framework of the right posterior section
To achieve R0 resection and spare more functional liver parenchyma, a tailored surgical procedure is necessary for safe ALR according to the morphological framework of the right posterior section (Figure 5). ARPS oriented by the RPPT ought to be performed in cases with enough lesion margin and functional liver remnant, no matter the morphological types. To achieve R0 resection, ARPS combined with dorsal subsegment 8 (S8d) resection oriented by the RPPT in the caudal part and the course of RHV in the cranial part is recommended for cases with poor lesion margin in type III and type IV. To maximize the functional liver remnant, parenchyma-sparing ARPS by reserving the variable Ventral-P6 is suitable for cases with poor liver function in type II and type IV. Parenchyma-sparing ARPS combined with S8d resection instead of ARPS is recommended for type IV cases with poor cut margin and poor liver function.

Validation of the conceptual framework
A total of 6 consecutive patients with lesions in the right posterior section who underwent tailored ALR under the guidance of 3D simulation and the proposed conceptual framework were prospectively enrolled during the study period. There were 5 males and 1 female, with an average age of 61.3±12.2 years. All patients were of Child-Pugh grade A and ICG-R15 <10%. ICG fluorescence imaging was utilized in 4 patients who underwent laparoscopic tailored ALR. Table 2 provides details of baseline characteristics and surgical outcomes of all HCC patients. Complete tumor removal (R0 resection) was achieved in all patients with a median tumor-free margin of 17.5 (6.5–25.5) mm. The average operation time was 303.7±116.2 min. The average blood loss was 509.2±179.2 mL, and 3 cases accepted blood transfusion. The mean postoperative hospital stay time was 10.5±4.2 days. Minor complication (pulmonary infection) that was transient and improved with conservative treatments occurred in 1 patient; there were no postoperative 90-day deaths. The proposed framework provided instructions on tailored ALR for HCC by maximizing tumor removal as well as maximizing functional liver remnant (Table 2).
Table 2
| Case | Sex | Age (yrs) | Type | Merit* | Surgical strategy | Surgical procedure | Previous abdominal surgery | Tumor size (cm) | Resection margin (mm) | Liver cirrhosis | Vascular invasion | Hospital stay (days) | Operation time (min) | Blood loss (ml) | Blood transfusion | Complication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 54 | III | A | ARPS + S8d | Open | Yes | 14.5 | 15 | Yes | Micro | 15 | 165 | 480 | Yes | None |
| 2 | Male | 81 | III | A | ARPS + S8d | Open | Yes | 5.5 | 5 | Yes | Micro | 15 | 190 | 350 | No | Pulmonary infection |
| 3 | Male | 66 | I | None | ARPS | Laparoscopic | No | 7.0 | 20 | Yes | None | 4 | 300 | 400 | No | None |
| 4 | Male | 54 | IV | A+B | Parenchyma-sparing ARPS + S8d | Laparoscopic | Yes | 3.5 | 33 | Yes | Micro | 8 | 480 | 375 | No | None |
| 5 | Male | 47 | I | None | ARPS | Laparoscopic | No | 9.0 | 7 | No | Micro | 11 | 320 | 800 | Yes | None |
| 6 | Female | 66 | IV | A+B | Parenchyma-sparing RPS + S8d | Laparoscopic | No | 6.3 | 23 | No | Micro | 10 | 367 | 650 | Yes | None |
*, A: obtain wider tumor-free resection margin; B: preserve more remnant liver volume. HCC, hepatocellular carcinoma; ARPS, anatomical right posterior sectionectomy; S8d, dorsal subsegment 8 resection; 3D, three-dimensional.
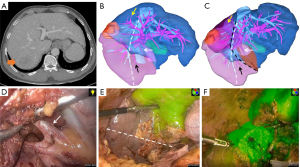
One exemplar case report is presented in Figure 6. We performed a tailored laparoscopic parenchyma-sparing RPS combined with S8d resection for a 54-year-old man with recurrent HCC in the right posterior section and type IVb variant using anterior approach technique and RHV-orientation based on ICG fluorescence imaging. Detailed patient characteristics are summarized in Video S1 (supporting information).
Discussion
Liver transection along the intersegmental borders of the liver is an essential technique for ALR. Systematic removal of the target portal territory and exposure of the landmark vein on the transection plane are two established determinants that indicate a precise ALR (7). Parenchymal transection guided by the demarcation line after clamping of the RPP and the plane of RHV was a standardized surgical procedure for ARPS. Nevertheless, right posterior portal branches vary considerably and the RHV does not always run along the right intersectional plane (16,17). In this study, we demonstrated that the transection plane determined by the RPPT was dissociated with the plane of RHV in approximately 60% of the present cases. Thereafter, the problem is how to define the precise direction and course of parenchymal transection in these dissociated cases, especially in the era of real-time fluorescence navigation with ICG for liver surgery (18). To resolve this issue, we herein proposed a novel comprehensive framework of the right posterior section for tailored and safe ALR based on a 3D simulation system, and classified the dissociation into three variants: caudal-redundant (type II), cranial-deficient (type III), and combined (type IV) variants.
In type II (caudal-redundant type), part of segment 6 tilted toward the ventral side of RHV after clamping or staining of the RPP. In the authors’ series, the aberrant Ventral-P6 (type IIa) or the distal portal branch of P6 running across the ventral side of RHV (type IIb) accounted for approximately 70% and 30% of this variant, respectively. Several clinical and surgical implications favor the thorough understanding of the type II variant. Firstly, almost all of the Ventral-P6 ramifies from the RPP near the hepatic hilum (type IIa). It can be easily approached and preserved after minor parenchymal transection along the RPP at the level of Rouviere’s sulcus. However, the deep-located Ventral-P6 in type IIa and the distal portal branch of P6 in type IIb can scarcely be approached and preserved (19). Moreover, the mean volume proportion supplied by Ventral-P6 in type IIa was up to 7.0%, significantly greater than that in type IIb (P<0.001). Accordingly, the RPP should be transected after meticulous dissection with preserving the Ventral-P6 in type IIa to spare more liver parenchyma for cases with poor liver function. Secondly, the anterior approach, in which the parenchymal transection is undertaken prior to the mobilization of the right liver, represents a superior surgical strategy that minimizes mechanical manipulation of tumor-bearing liver and potentially reduces hematogenous dissemination of tumor cells as compared with the conventional approach in right hepatectomy (20). However, full mobilization and medial rotation of the right liver have been the conventional or standard approach that allows access to the dorsolaterally located ischemic demarcation. So, the application of anterior approach surgical technique has rarely been described in ARPS (21). We have emphasized that the caudal part of the RPPT-oriented transection plane is ventrally tilted against the course of the RHV trunk in type II. Therefore, the type II variant allows for parenchymal transection from the caudal to the cranial side with peripheral-to-central dissection before the right liver is mobilized, and represents an anatomical hallmark for the use of anterior approach technique in ARPS.
A possible dissociation between the cranial part of the RPPT-oriented transection plane and the course of the RHV trunk (cranial-deficient variant, type III) has been mentioned in a few articles (7,22). We demonstrated two subtypes of portal pedicle variation in type III that required ARPS or other alternative tailored ALR as shown in Figure 5. The aberrant RP-7 (type IIIa) or the P8c crossing over to the RHV (type IIIb) accounted for approximately 40% and 60% of this variant, respectively. It was reported by Hosokawa et al. that the right subphrenic region posterior to the RHV was fed by P8c in 85% of the 100 cases who underwent left trisectionectomy (23). In type IIIa, the RP-7 can easily be approached after minor parenchymal transection along the RP, which is similar to that of type IIa. By contrast, the deep-located P8c across the RHV in type IIIb can scarcely be approached, which is similar to that of type IIb. Moreover, we found that the median volume proportion supplied by the aberrant RP-7 in type IIIa was up to 12.0%, significantly greater than that supplied by the aberrant P8c in type IIIb (P<0.001). Accordingly, ARPS guided by the RPP-oriented ICG fluorescence navigation, rather than the landmark RHV trunk, may enhance the safety of the parenchyma sparing principle for patients with the type IIIa variant when sufficient surgical margins can be secured. Nevertheless, exposure of the landmark RHV trunk on the cut surface remains an important technique for avoiding disorientation and maximizing surgical margins during ARPS, especially in the absence of ICG fluorescence navigation (7). ARPS combined with S8d resection instead of APPS is recommended for cases with type IIb variant to obtain R0 resection.
Limitation
The main limitation of this study is that the proposed framework was merely validated in a single-center exploratory cohort with a limited sample size. The number of the clinical samples was too small to scientifically verify the effectiveness of the proposed framework by comparing cases using a 3D simulation system and those without this system. Multi-centered evaluation with relatively large sample size is warranted prior to the advocation of the proposed framework in routine clinical practice. We will focus on expanding the sample size and conducting a multi-center clinical controlled study to validate the feasibility of the proposed framework in further study. Furthermore, although we demonstrated that the proposed framework reconciled the parenchyma sparing principle and sufficient surgical margins with favorable short-term outcomes, no long-term results were provided to further support the superiority of the novel framework over the conventional concept for conducting ARPS due to the short observation period.
Conclusions
In conclusion, we propose a novel and comprehensive framework of the right posterior section that consists of four morphological types based on a 3D simulation system. Precise preoperative planning accompanied by an individualized surgical approach based on the proposed framework allows tailored territorial liver resections that enhance the safety and accuracy of ARPS or other alternative ALR.
Acknowledgments
Funding: This work was supported by Key Program of the National Natural Science Foundation of China (No. 31930020), National Natural Science Foundation of China (No. 82102150), Natural Science Foundation of Jiangsu Province (No. BK20210968), and Social Development Projects of Jiangsu Province (No. BE2020708).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1105/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1105/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1105/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1105/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (approval number: 2020-SR-444) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shindoh J, Makuuchi M, Matsuyama Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol 2016;64:594-600. [Crossref] [PubMed]
- Margonis GA, Buettner S, Andreatos N, et al. Anatomical Resections Improve Disease-free Survival in Patients With KRAS-mutated Colorectal Liver Metastases. Ann Surg 2017;266:641-9. [Crossref] [PubMed]
- Liao KX, Chen L, Ma L, et al. Laparoscopic middle-hepatic-vein-guided anatomical hemihepatectomy in the treatment of hepatolithiasis: a 10-year case study. Surg Endosc 2022;36:881-8. [Crossref] [PubMed]
- Jang JS, Cho JY, Ahn S, et al. Comparative Performance of the Complexity Classification and the Conventional Major/Minor Classification for Predicting the Difficulty of Liver Resection for Hepatocellular Carcinoma. Ann Surg 2018;267:18-23. [Crossref] [PubMed]
- Kawaguchi Y, Fuks D, Kokudo N, et al. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg 2018;267:13-7. [Crossref] [PubMed]
- Inoue Y, Arita J, Sakamoto T, et al. Anatomical Liver Resections Guided by 3-Dimensional Parenchymal Staining Using Fusion Indocyanine Green Fluorescence Imaging. Ann Surg 2015;262:105-11. [Crossref] [PubMed]
- Monden K, Alconchel F, Berardi G, et al. Landmarks and techniques to perform minimally invasive liver surgery: A systematic review with a focus on hepatic outflow. J Hepatobiliary Pancreat Sci 2022;29:66-81. [Crossref] [PubMed]
- Majno P, Mentha G, Toso C, et al. Anatomy of the liver: an outline with three levels of complexity--a further step towards tailored territorial liver resections. J Hepatol 2014;60:654-62. [Crossref] [PubMed]
- Mise Y, Hasegawa K, Satou S, et al. How Has Virtual Hepatectomy Changed the Practice of Liver Surgery?: Experience of 1194 Virtual Hepatectomy Before Liver Resection and Living Donor Liver Transplantation. Ann Surg 2018;268:127-33. [Crossref] [PubMed]
- Ichida H, Imamura H, Yoshioka R, et al. Re-evaluation of the Couinaud classification for segmental anatomy of the right liver, with particular attention to the relevance of cranio-caudal boundaries. Surgery 2021;169:333-40. [Crossref] [PubMed]
- Watanabe A, Yoshizumi T, Harimoto N, et al. Right hepatic venous system variation in living donors: a three-dimensional CT analysis. Br J Surg 2020;107:1192-8. [Crossref] [PubMed]
- Watanabe N, Ebata T, Yokoyama Y, et al. Anatomic features of independent right posterior portal vein variants: Implications for left hepatic trisectionectomy. Surgery 2017;161:347-54. [Crossref] [PubMed]
- Minami T, Ebata T, Yokoyama Y, et al. Study on the Segmentation of the Right Posterior Sector of the Liver. World J Surg 2020;44:896-901. [Crossref] [PubMed]
- Yamamoto Y, Sugiura T, Okamura Y, et al. The Pitfalls of Left Trisectionectomy or Central Bisectionectomy for Biliary Cancer: Anatomical Classification Based on the Ventral Branches of Segment VI Portal Vein Relative to the Right Hepatic Vein. J Gastrointest Surg 2017;21:1453-62. [Crossref] [PubMed]
- Takayasu K, Moriyama N, Muramatsu Y, et al. Intrahepatic portal vein branches studied by percutaneous transhepatic portography. Radiology 1985;154:31-6. [Crossref] [PubMed]
- Shindoh J, Mise Y, Satou S, et al. The intersegmental plane of the liver is not always flat--tricks for anatomical liver resection. Ann Surg 2010;251:917-22. [Crossref] [PubMed]
- Wakabayashi T, Benedetti Cacciaguerra A, Ciria R, et al. Landmarks to identify segmental borders of the liver: A review prepared for PAM-HBP expert consensus meeting 2021. J Hepatobiliary Pancreat Sci 2022;29:82-98. [Crossref] [PubMed]
- Nishino H, Seo S, Hatano E, et al. What is a precise anatomic resection of the liver? Proposal of a new evaluation method in the era of fluorescence navigation surgery. J Hepatobiliary Pancreat Sci 2021;28:479-88. [Crossref] [PubMed]
- Yamamoto M, Katagiri S, Ariizumi S, et al. Tips for anatomical hepatectomy for hepatocellular carcinoma by the Glissonean pedicle approach (with videos). J Hepatobiliary Pancreat Sci 2014;21:E53-6. [Crossref] [PubMed]
- Jabir MA, Hamza HM, Fakhry H, et al. Anterior Versus Conventional Approach for Resection of Large Right Lobe Hepatocellular Carcinoma. J Gastrointest Cancer 2017;48:25-30. [Crossref] [PubMed]
- Ferrero A, Lo Tesoriere R, Giovanardi F, et al. Laparoscopic right posterior anatomic liver resections with Glissonean pedicle-first and venous craniocaudal approach. Surg Endosc 2021;35:449-55. [Crossref] [PubMed]
- Li J, Li X, Zhang X, et al. Indocyanine green fluorescence imaging-guided laparoscopic right posterior hepatectomy. Surg Endosc 2022;36:1293-301. [Crossref] [PubMed]
- Hosokawa I, Ohtsuka M, Yoshitomi H, et al. Right intersectional transection plane based on portal inflow in left trisectionectomy. Surg Radiol Anat 2019;41:589-93. [Crossref] [PubMed]


