
Thyroid autoantibody distribution in patients with latent autoimmune diabetes in youth: a multicenter, national survey
Introduction
Type 1 diabetes mellitus (T1DM) is generally divided into rapidly progressive insulin-dependent diabetes mellitus (RPIDDM) and slowly progressive insulin-dependent diabetes mellitus (SPIDDM) according to the speed of progression of beta cell autoimmunity. SPIDDM is also named latent autoimmune diabetes (LAD), “hybrid diabetes”, “type 1.5 diabetes (T1.5D)” and “double diabetes (DD)” (1,2). Regarding onset age, LAD is grouped into LAD in youth (LADY) and LAD in adults (LADA); the most widely used cutoff age between LADY and LADA is 30 years, as defined by the Immunology of Diabetes Society (IDS) in 2005 (3). Compared with LADA patients, LADY patients have received insufficient attention, especially in China, and many related publications are case reports or small sample studies (4-6). The prevalence of islet autoantibodies in youth type 2 diabetes mellitus (T2DM) patients ranges from 1.05% to 75% in different countries and populations (7). The onset characteristics of LADY are similar to those of LADA and include noninsulin-dependent diabetes with beta cell autoantibodies and similar early clinical manifestations to T2DM. Studies have shown important differences in insulin sensitivity, insulin secretion metabolism, therapy method, susceptible human leukocyte antigen (HLA) genetic load and cytokine levels between LADY and youth T2DM patients (8-10), which deepens our knowledge of LADY. However, few studies have reported the incidence of comorbidities in LADY patients.
The link between DM and thyroid disease has long been recognized. Many studies have described a higher incidence of thyroid disease in DM patients than in the general population, and autoimmune thyroid disease (AITD) is the most common autoimmune disorder coexisting with DM, particularly with autoimmune DM (ADM) (11,12). AITD is characterized by the presence of thyroid peroxidase autoantibody (TPOA) and thyroglobulin autoantibody (TGA); similarly, a higher frequency of TPOA or TGA indicates a higher risk of AITD (13). According to our estimates based on observations over recent years, the seroprevalence rates of positive thyroid autoantibodies in DM patients are 12.3–27.2% in patients with younger T1DM (14-16), 10–32% in patients with LADY (17,18), 24.4–35.8% in patients with older T1DM (19,20), 16.3–41% in patients with LADA (11,21,22) and 8–45% in patients with T2DM (17,23). Among those reports, reports on LADY are extremely scarce, and none of them are from the Chinese population, in which the base of ADM is the largest and the genetic background is different from that of the Caucasians population. Simultaneously, the thyroid autoantibodies positive rate is closely related to sex and glutamic acid decarboxylase (GADA) titers (21). Thus, studying the prevalence of thyroid autoantibodies in LADY patients and stratifying patients according to sex and GADA titers are crucial for prioritizing the clinical screening of patients who are prone to AITD.
Therefore, in this multicenter and national study, we attempted to analyze the thyroid autoantibodies prevalence in patients with LADY to clarify the necessity of thyroid autoantibody detection and provide a basis for the clinical screening of AITD by stratifying sex and GADA titers. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-423/rc).
Methods
Subjects
This study involved 17,753 DM patients aged 15–79 years who were newly diagnosed with diabetes between 2015 and 2017 (Figure 1). All subjects were Chinese and were recruited from 46 tertiary hospitals located in 25 cities throughout the entire country. Training was given to relevant staff in each hospital, and all data were collected using standardized procedures and methods (24). The inclusion criteria in the current study were as follows: (I) diagnosis age ranging from 15 to 79; (II) duration of diabetes <1 year; and (III) in the endocrinology clinic of the hospital for treatment. The exclusion criteria were as follows: (I) patients with special DM types, such as pregnancy at diagnosis or gestational diabetes mellitus (GDM) and fulminant T1DM (FT1DM); (II) patients with coexisting acute diseases that could influence glucose metabolism, such as stress and infection; and (III) patients with tumors or severe diseases.
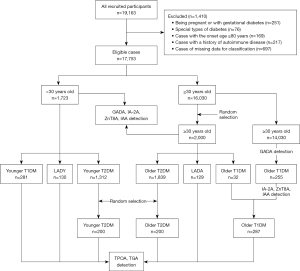
Ultimately, 3,723 patients (1,723 patients <30 years old and 2,000 patients ≥30 years old) were included in the current study. Among them, patients ≥30 years old were selected by a random sampling method in IBM SPSS 25.0. All 3,723 patients had autoantibodies against GADA, insulinoma-associated-2 (IA-2A), zinc transporter-8 (ZnT8A) and insulin autoantibody (IAA). Because IAA is indistinguishable from insulin antibody (IA) upregulation after insulin treatment, IAA was only detected in subjects who were not taking insulin. In terms of the islet autoantibody results, clinical features, and age, 3,723 patients were grouped into 6 DM subtypes by selection and sampling: younger T1DM (n=281), LADY (n=130), younger T2DM (n=200, randomly drawn from 1,312 subjects), older T1DM [n=287; note: Because of the low incidence of T1DM in older patients (only 32 out of the 2,000 older patients sampled were T1DM patients), so we included all T1DM patients with a diagnosis age ≥30 years based on GADA test results and other T1DM diagnostic criteria mentioned below, to make the number of older T1DM patients equal to that of patients in other DM groups], LADA (n=129), and older T2DM (n=200, randomly selected from 1,839 patients). Thyroid autoantibodies (TPOA and TGA) were tested in all 6 groups of patients. A diagram detailing the study enrollment and assays is presented in Figure 1.
The diagnostic criteria for the different DM subtypes were as follows. T1DM: (I) insulin dependent after diagnosis; (II) diagnosed by two independent endocrinologists and met at least one of the following criteria: fasting C-peptide (FCP) level <200 pmol/L, positive for any islet autoantibody, typical symptoms of metabolic disturbances related to diabetes, and diabetic ketosis or ketoacidosis. LADY: (I) diagnosis age <30 years; (II) positivity for any islet autoantibody; and (III) insulin independence (insulin use <1 month) at least 6 months after diagnosis. LADA: (I) diagnosis age ≥30 years; (II) positivity for any islet autoantibody; and (III) insulin independence for 6 or more months since diagnosis. T2DM: (I) meeting the diagnostic criteria for diabetes set by the World Health Organization (WHO) in 1999; and (II) negative for four islet autoantibodies. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (Approval No. [2014] Lun Shen [Division] No. 32), and all participants or their guardians provided written informed consent forms.
Autoantibody assays
Serum TPOA and TGA were detected by chemiluminescence (Siemens Healthcare Diagnostics Inc., Germany) with a cutoff value of 60 U/mL. The radioligand assays (RLAs) method was used to test the seropositivity of GADA, IA-2A and ZnT8A and the electrochemiluminescence (ECL) method was used to detect the seropositivity of IAA. The sensitivity and specificity of the methods were consistent with our previous study (25). According to the data of the 99th percentile in 405 healthy subjects, the critical value of IA-2A was 3.3 U/mL and that of GADA was 18 U/mL (U is a WHO unit), of which, high GADA titers were defined as ≥180 U/mL (26). Threshold autoantibody indicators were 0.011 for ZnT8A and 0.005 for IAA.
Clinical data and biochemical assays
Body weight, height, and blood pressure were recorded locally. Serum was assayed on an automatic chemistry analyzer to measure the levels of fasting blood sugar (FBS), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL), and low-density lipoprotein cholesterol (LDL). The levels of FCP and postprandial C-peptide (PCP) in serum were determined by chemiluminescence using a commercially available kit (Advia Centaur System, Siemens). For the C-peptide test, the intra-assay variation coefficients were 1.0–3.3% and the interassay variation coefficients were 3.7–4.1%. HbA1c (glycated hemoglobin) was determined via automated liquid chromatography (Bio-Rad VARIANT-II Hemoglobin Testing System, United States). FBS, lipids, HbA1c and FCP were tested with overnight fasting venous blood samples; and the levels of PCP were detected with 2-hour postprandial blood samples.
Statistical analysis
All statistical analyses were conducted with IBM SPSS 25.0. Q–Q plots were used to verify the continuous variables of normality. Normal distributed variables were expressed as the mean ± SD, while abnormal distribution variables were presented as the median (interquartile range). Or, some of the continuous variables were binarized and were shown as positive cases numbers, ratios, or composition ratios. Classified variables were presented as percentages (number, n). Analyses of continuous variables were conducted with single factor analysis of variance (ANOVA) or nonparametric tests. The χ2 test or Fisher’s exact test was used to compare frequencies. Multiple linear regression was used to adjust for potential confounders. P<0.05 (two-tailed) was considered significant for all tests.
Results
Prevalence of thyroid autoantibodies in LADY and other DM subtypes
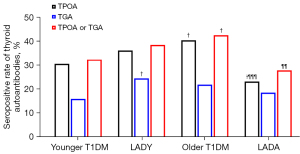
The prevalence of thyroid autoantibodies in patients with LADY was 36.2% for TPOA, 24.6% for TGA and 38.5% for TPOA or TGA (Table S1). As expected, the frequencies of thyroid autoantibodies in LADY patients were higher than those in younger T2DM patients (Table S1); after adjusting for age and sex, compared with younger T2DM patients, the risk of thyroid autoantibodies in LADY patients was 6.85-fold for TGA, 6.43-fold for TPOA, and 6.64-fold for TGA or TPOA (P<0.001 for all, Table 1). Notably, the seropositive rate of TGA was higher in LADY patients than that of younger T1DM patients (24.6% vs. 16%, respectively; P=0.038) (Figure 2), but after adjusting for age and sex, the difference was no longer significant (P=0.066) (Table 1). The frequency of TPOA positivity in LADY patients was higher than that in LADA patients (36.2% vs. 23.3%, respectively; P=0.023) (Figure 2). The results of multivariable analysis (adjusted for sex and GADA positivity) showed that LADY patients had a 1.94-fold increased likelihood of positivity for TPOA than that of LADA patients (OR =1.94, P=0.023) (Table 1).
Table 1
| Variables | LADY vs. younger T1DM | LADY vs. LADA | LADY vs. younger T2DM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ORa | 95% CI | P value | ORb | 95% CI | P value | ORa | 95% CI | P value | |||
| TGA | 1.64 | 0.97–2.77 | 0.066 | 1.43 | 0.76–2.70 | 0.270 | 6.85 | 3.13–14.99 | <0.001 | ||
| TPOA | 1.23 | 0.79–1.93 | 0.364 | 1.94 | 1.09–3.42 | 0.023 | 6.43 | 3.43–12.07 | <0.001 | ||
| TGA/TPOA | 1.24 | 0.79–1.93 | 0.345 | 1.70 | 0.97–2.95 | 0.062 | 6.65 | 3.59–12.30 | <0.001 | ||
ORa, adjusted for age and sex; ORb, adjusted for sex, GADA- and IA-2A-positivity. LADY, latent autoimmune diabetes in youth; T1DM, type 1 diabetes mellitus; LADA, latent autoimmune diabetes in adults; T2DM, type 2 diabetes mellitus; OR, odds ratio; TGA, thyroglobulin autoantibody; TPOA, thyroid peroxidase autoantibody.
To analyze the effect of sex on the prevalence of thyroid autoantibodies, we divided patients with the 4 ADM types into two groups by sex. The results showed that the positive rate of thyroid antibodies was higher in females than in males in patients with younger T1DM and LADA patients (P<0.05) (Figure S1A); in LADY and older T1DM patients, limited by the sample size, no significant differences were shown (Figure S1A). For T1DM patients, whether the patients were male or female, the prevalence of TPOA was higher than that of TGA (P≤0.05); for LADY patients, TPOA positivity was higher than TGA in male patients only (P=0.034) (Figure S1B,S1C).
The association between islet autoantibodies and thyroid autoantibodies in LADY and other ADM subtypes
Among T1DM patients, GADA- and IA-2A-positive patients were more likely to present seropositivity for TPOA or TGA than GADA- and IA-2A-negative patients, while such associations between thyroid autoantibodies and ZnT8A were only shown in older T1DM patients (Figure S2). For LAD patients, although there were no differences in thyroid autoantibody frequencies between GADA-, IA-2A- and ZnT8A-positive and -negative patients, a potential association seemed to exist between GADA-positive and TGA-positive patients (for patients with LADY, P=0.064; for patients with LADA, P=0.062) (Figure S2). Moreover, LADY patients with a high GADA titer more frequently expressed thyroid autoantibodies than LADY patients with a low GADA titer (47.2% vs. 19.4%, respectively, P=0.005 for TPOA; 34.2% vs. 13.9%, P=0.023 for TGA; 51.4% vs. 22.2%, P=0.004 for TPOA or TGA) (Figure 3A). When we stratified the patients by sex, the results showed that only in males (n=69) patients with high GADA titers have a higher prevalence of TPOA or TGA than patients with low GADA titers did (Figure 3B,3C). The association between high GADA titers and thyroid antibody positivity remained significant after adjustment for age (OR =11.14, P=0.025 for TGA; OR =4.99, P=0.011 for TPOA; OR =5.52, P=0.007 for TPOA or TGA) (Table 2). Unexpectedly, LADY patients with IAA negativity were more likely to present TGA positivity than LADY patients with IAA positivity, which may have been due to the limited sample size (Figure S2).
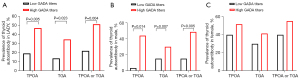
Table 2
| Variables | ORa | 95% CI | P value |
|---|---|---|---|
| TGA | 11.14 | 1.35–91.85 | 0.025 |
| TPOA | 4.99 | 1.44–17.20 | 0.011 |
| TGA/TPOA | 5.52 | 1.60–19.10 | 0.007 |
ORa, adjusted for age, high vs. low GADA titers. LADY, latent autoimmune diabetes in youth; GADA, glutamic acid decarboxylase autoantibody; OR, odds ratio; TGA, thyroglobulin autoantibody; TPOA, thyroid peroxidase autoantibody.
Clinical features of LADY patients with and without thyroid autoantibodies
Age, body mass index (BMI), FBS, FCP, PCP, HbA1c, and serum lipids levels were compared between TPOA- or TGA-positive (n=50) and TPOA- and TGA-negative (n=80) patients with LADY. Compared to those without thyroid autoantibodies, TPOA- or TGA-positive patients with LADY were diagnosed at later ages, had higher HDL levels, lower BMIs and TG levels. No significant differences were found for sex, blood pressure, or FBS, HbA1c, CP, TC or LDL levels between the two groups (Table S2).
Clinical features in LADY and other DM subtypes
Among younger patients, those with LADY were older than those with younger T1DM patients (24.51±4.20 vs. 23.02±4.48, respectively; P<0.001) (Table 3). The levels of FCP were highest in patients with younger T2DM (496.2 pmol/L; 95% CI: 266.4–750.7 pmol/L) and lowest in patients with younger T1DM (102.9 pmol/L; 95% CI: 40.0–186.5 pmol/L) and those in patients with LADY (281.0 pmol/L; 95% CI: 168.4–484.8 pmol/L) were between the highest and the lowest (P<0.001 for all); PCP levels and BMI exhibited a similar trend (P<0.001 for all) (Table 3). Moreover, HbA1c levels in LADY patients [10.97% (± SD: 3.33%)] were lower than those in younger T1DM patients [11.69% (± SD: 3.48%)] (P<0.05); in contrast, the frequency of GADA positivity in LADY patients was higher than that in younger T1DM patients (83.1% vs. 68.3%, respectively; P<0.01) (Table 3). Moreover, no differences were found in lipid levels between younger T1DM and LADY patients (Table 3).
Table 3
| Variables | Younger T1DM (n=281) | LADY (n=130) | Younger T2DM (n=200) | Older T1DM (n=287) | LADA (n=129) | Older T2DM (n=200) |
|---|---|---|---|---|---|---|
| Age (years) | 23.02±4.48 | 24.51±4.20††† | 24.07±4.53† | 47.44±10.58†††ⱡⱡⱡ§§§ | 53.07±11.59†††ⱡⱡⱡ§§§¶¶¶ | 52.30±11.74†††ⱡⱡⱡ§§§¶¶¶ |
| Female | 39.9% (112/281) | 37.7% (49/130) | 35.0% (70/200) | 40.1% (115/287) | 44.2% (57/129) | 37.0% (74/200) |
| BMI (kg/m2) | 19.97±3.61 | 21.98±4.01††† | 25.06±4.40†††ⱡⱡⱡ | 21.48±3.76†††§§§ | 23.26±3.54†††ⱡⱡ§§¶¶¶ | 25.15±3.89†††ⱡⱡⱡ¶¶¶*** |
| SBP (mmHg) | 115.62±13.58 | 116.96±13.62 | 120.51±12.51††† | 121.07±16.71††† | 124.57±14.62†††ⱡⱡⱡ | 128.84±16.53†††ⱡⱡⱡ§§§¶¶¶ |
| DBP (mmHg) | 73.61±10.43 | 74.66±9.35 | 76.94±9.70†† | 76.16±11.43 | 78.86±9.99†††ⱡⱡ | 88.67±11.06†††ⱡⱡⱡ¶ |
| FBS (mmol/L) | 9.70±4.56 | 9.32±4.37 | 9.19±3.46 | 9.63±4.41 | 9.68±4.10 | 9.31±3.45 |
| HbA1c (%) | 11.69±3.48 | 10.97±3.33† | 10.57±2.68††† | 11.25±2.87 | 10.05±2.86†††¶¶¶ | 9.25±2.75†††ⱡⱡⱡ§§§¶¶¶ |
| FCP (pmol/L) | 102.9 (40.0–186.5) |
281.0 (168.4–484.8)††† |
496.2 (266.4–750.7)†††ⱡⱡⱡ |
100.0 (33.3–200.0)ⱡⱡⱡ§§§ |
467.9 (283.5–724.5)†††ⱡⱡⱡ¶¶¶ |
516.2 (359.8–830.6)†††ⱡⱡⱡ¶¶¶ |
| PCP (pmol/L) | 180.0 (86.6–366.0) |
559.4 (338.7–1033.1)††† |
1070.0 (599.9–1733.2)†††ⱡⱡⱡ |
178.5 (66.6–451.3)ⱡⱡⱡ§§§ |
1115.0 (571.9–1601.3)†††ⱡⱡⱡ¶¶¶ |
1449.9 (942.4–2165.5)†††ⱡⱡⱡ§¶¶¶** |
| GADA | 68.3% (192/281) | 83.1% (108/130)†† | – | 65.6% (188/287)ⱡⱡⱡ | 72.1% (93/129)ⱡ | – |
| IA-2A (%) | 40.2 (113/281) | 31.5 (41/130) | – | 25.4 (73/287)††† | 14 (18/129)†††ⱡⱡⱡ¶¶ | – |
| ZnT8A (%) | 24.9 (70/281) | 31.5 (41/130) | – | 16 (46/287)††ⱡⱡⱡ | 31 (40/129)¶¶¶ | – |
| IAA (%) | 15.2 (5/33) | 20.3 (12/59) | – | 15.8 (6/38) | 14.1 (9/64) | – |
| At least two Ab positive (%) | 42.7 (120/281) | 43.8 (57/130) | – | 29.3 (84/287)†††ⱡⱡⱡ | 17.1 (22/129)†††ⱡⱡⱡ¶¶ | – |
| TG (mmol/L) | 0.99 (0.75–1.56) | 1.21 (0.83–2.02) | 1.93 (1.10–3.40)†††ⱡⱡⱡ | 1.10 (0.74–1.84)§§§ | 1.46 (0.96–2.16)†††§¶ | 1.71 (1.24–2.46)†††ⱡⱡⱡ¶¶¶ |
| TC (mmol/L) | 4.25±1.25 | 4.42±1.22 | 4.68±1.48††† | 4.50±1.50 | 4.61±1.22† | 4.64±1.42†† |
| HDL-C (mmol/L) | 1.19 (0.98–1.49) | 1.11 (0.96–1.35) | 1.00 (0.87–1.24)†††ⱡ | 1.19 (0.97–1.48)§§§ | 1.18 (0.94–1.43)§§ | 1.12 (0.94–1.32)§ |
| LDL-C (mmol/L) | 2.53±0.89 | 2.73±1.03 | 2.71±0.96 | 2.66±1.05 | 2.79±1.08 | 2.81±0.98††¶ |
| Antihypertensive medication | 0.71% (2/281) | 0.77% (1/130) | 3% (6/200) | 9.1% (26/287)†††ⱡⱡⱡ§§ | 20.2% (26/129)†††ⱡⱡⱡ§§§¶¶ | 17% (34/200)†††ⱡⱡⱡ§§§¶¶ |
| Lipid drug | 0.71% (2/281) | 5.4% (7/130)† | 9.5% (19/200)††† | 8.7% (25/287)††† | 10.9% (14/129)††† | 14% (28/200)†††ⱡ |
Data are presented as medians (interquartile ranges), means ± standard deviations, or ratios and their numbers where appropriate. †P<0.05, ††P≤0.01, †††P≤0.001 compared with younger T1DM patients; ⱡP<0.05, ⱡⱡP≤0.01, ⱡⱡⱡP≤0.001 compared with LADY patients; §P<0.05, §§P≤0.01, §§§P≤0.001 compared with younger T2DM patients; ¶P<0.05, ¶¶P≤0.01, ¶¶¶P≤0.001 compared with older T1DM patients; **P≤0.01, ***P≤0.001 compared with LADA patients. T1DM, type 1 diabetes mellitus; LADY, latent autoimmune diabetes in youth; T2DM, type 2 diabetes mellitus; LADA, latent autoimmune diabetes in adults; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; HbA1c, glycated hemoglobin; FCP, fasting C-peptide; PCP, postprandial C-peptide; GADA, glutamic acid decarboxylase; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
For LAD, in addition to age, the levels of blood pressure, BMI, FCP, and PCP in LADA patients were all higher than those in LADY patients; in contrast, the prevalence of GADA, IA-2A and at least two autoantibodies were higher in LADY patients than those of LADA patients (Table 3). The frequencies of GADA, ZnT8A and positivity for at least two autoantibodies were higher in younger patients than those of older patients (Table 3).
Discussion
In the past, the study of our team mainly concentrated on the LADA or T1DM patients. Our previous study found that LADA or T1DM patients have an increased risk of developing organ-specific autoimmune diseases, and a strong link was observed between thyroid autoantibody positivity and GADA or IA-2A positivity (27). Recently, we began to pay attention to LADY patients. We have explored the similarities and differences in clinical manifestations, inflammatory factors and genetics between LADY and type 2 diabetes (10). However, the positive rate of thyroid autoantibodies in LADY patients has not been reported in China. In this multicenter and national study, the results mainly demonstrated the importance of screening for thyroid autoantibodies in LADY patients due to their high prevalence. We also recommend that special clinical attention be paid to the thyroid autoantibody status of male LADY patients with high GADA titers to identify patients at high risk of developing AITD in a timely manner.
The positive frequencies of thyroid autoantibodies in DM patients are affected by many factors, such as age, sex, DM subtype, GADA positivity and GADA titer. A high seropositive rate of thyroid autoantibodies is often reported in patients with T1DM, LADA and T2DM. In the present study, we found that the prevalence of TGA or TPOA in LADY patients was similar to that in younger T1DM patients after adjusting for age and sex. As mentioned above, the prevalence of thyroid autoantibodies increases with advancing age. Therefore, univariate analysis showed that the positive rate of TGA in LADY patients was higher than that of young T1DM patients, which may be related to the older age of LADY patients. However, even after adjusting for sex, GADA- and IA-2A- positivity, patients with LADY had a 1.94-fold higher risk of TPOA than those with LADA patients, who had an older age of diagnosis. We speculated that a specific genetic risk marker may be active in LADY patients. In current study, the thyroid autoantibody prevalence in LADY patients was also higher than that in LADY patients of USA (10%) (28) and Germany (32%) (17), which may be attributed to the genetic background, diagnostic criteria, sample size and advances in thyroid autoantibody detection technology in recent years. As expected from previous studies, as a subtype of ADM, the incidence of positive thyroid autoantibodies in patients with LADY was absolutely higher than that in patients with nonADM (younger and older T2DM patients). In brief, this high prevalence of TPOA or TGA in LADY patients indicates the necessity of thyroid autoantibodies screening in younger T2DM patients with islet autoantibody positivity for early identification of patients at high risk of AITD, as organ-specific autoantibodies in ADM can help identify patients at risk of developing other organ-specific autoimmune disorders (2). Follow-up studies of patients with positive thyroid antibodies are necessary to determine whether the incidence of AITD in LADY patients is also higher than that in LADA patients.
We also performed a comparison on the frequency of positive overlap among islet autoantibodies and thyroid autoantibodies among 4 ADM patients. The results suggested that T1DM patients with GADA-, IA-2A- and ZnT8A-positivity were more likely to exhibit thyroid autoantibody positivity, which is consistent with some Western studies (14,29). However, the results were inconsistent with our previous results (27), possibly because the subjects included in the study were different: in the present study, the T1DM patients were ≥30 years old, and in the previous study, the ADM patients (including T1DM and LADA patients) were ≥20 years old. In addition, the current study was limited by the sample size; although the difference was not significant in LADY and LADA patients, a higher thyroid autoantibody prevalence trend was still observed in patients who were positive for islet autoantibodies compared with patients who were negative for islet autoantibodies. In terms of previous studies, thyroid autoantibody positivity was more common in LADA patients with high GADA titers, and epitope spreading may be one of the possible mechanisms for the increased frequency of related antibodies (21,30). When we divided patients of LADY into two groups according to the GADA titers, we found that patients with high GADA titers presented a higher frequency of TPOA or TGA positivity than patients with low GADA titers. Further analysis showed that, after adjusting for age, male LADY patients with high GADA titers had an 11.14-fold, 5-fold and 5.52-fold higher risk of TGA, TPOA and TGA/TPOA positivity, respectively, than male patients with low GADA titers. Sex bias has also been found in LADA patients with high GADA titers which indicated that the autoimmune background of patients with high GADA titers may act as a ‘promoter’ for the spread of specific thyroid epitopes in male patients (21). These findings suggested a remarkably increased risk of a coexistent AITD in male LADY patients with higher GADA titers. Moreover, the comparison of the clinical features of LADY patients with and without thyroid antibodies showed that it is almost impossible to differentiate LADY patients who are with positive and negative for thyroid autoantibodies by clinical features, which further stresses the necessity of TPOA or TGA screening.
International reports comparing the clinical characteristics of LADY and other DM subtypes, especially ADM, are very limited. In our study, on average, LADY patients presented an older diagnosis age than younger T1DM patients. There are two possible reasons for this difference. First, patients with T1DM are usually diagnosed by typical onset symptoms, such as ketosis or ketoacidosis. In contrast, the onset of LAD patients is more insidious, usually diagnosed through a health examination or by chance during hospital visits for other diseases. Therefore, the difference in onset characteristics may result in a longer interval between the age at onset and the age at diagnosis in LADY patients than in T1DM patients; that is, the onset age of LADY patients may be older than that of T1DM patients. Second, the difference may be attributed to the lower genetic contribution of HLA according to the “threshold hypothesis” (31), which suggests that type 1 diabetes is a result of the combined contribution of genetics and the environment that exceeds the disease threshold. This result is consistent with a small sample study in the US (18); however, the US study also showed an age difference between LADY and younger T2DM patients, which was not shown in our study or others studies (32,33). Moreover, in our study, patients with LADY had a higher BMI, better blood glucose control, and better islet function than patients with younger T1DM (lipid levels were perhaps influenced by treatment). However, when compared with LADA patients, LADY patients were leaner and had worse blood glucose control and islet function (blood pressure was perhaps influenced by treatment). Therefore, in terms of clinical features, LADY may be a DM type that in different from younger T1DM and LADA.
In summary, our study suggested that routine screening for thyroid autoantibodies should be recommended for LADY patients, at the same time, we emphasized that special clinical attention should be paid to the thyroid autoantibody status of male LADY patients with high GADA titers to identify patients at high risk of AITD early. The limitation of the current study is that it was a cross-sectional study; a large-sample follow-up study is necessary to study the prevalence of AITD in LADY patients and verify whether LADY patients with thyroid autoantibodies are truly at high risk of developing thyroid dysfunction and the clinical manifestation of AITD.
Acknowledgments
The authors thank all patients, nurses, doctors, investigators, and technicians involved at the 46 participating centers of National Clinical Research Center for Metabolic Diseases for their efforts in data and sample collection (see https://cdn.amegroups.cn/static/public/atm-22-423-1.pdf). We also thank Prof. Guang Ning for his kind help in providing the population census data of the general population in China in 2010. We thank Prof. Bill Hagopian for providing the recombinant hGAD65 and IA-2 plasmid, and thank Prof. John C. Hutton for providing the recombinant ZnT8 plasmid. We also thank Prof. Yu Liping for technical support of IAA test based on electrochemiluminescence.
Funding: This study was supported by the Chinese National Key Research and Development Project (No. 2018YFC1315600), the National Key R&D Program of China (Nos. 2018YFC1315603, 2013BAI09B12, 2016YFC1305001), the National Natural Science Foundation of China (No. 81820108007), Science and Technology Major Project of Hunan Province (No. 2017SK1020), National Science and Technology Major Project (No. 2020ZX09201-028), the National Research and Development Program of China (No. 2018YFC2001005).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-423/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-423/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-423/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pozzilli P, Guglielmi C, Caprio S, et al. Obesity, autoimmunity, and double diabetes in youth. Diabetes Care 2011;34:S166-70. [Crossref] [PubMed]
- Leslie RD, Palmer J, Schloot NC, et al. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia 2016;59:13-20. [Crossref] [PubMed]
- Fourlanos S, Dotta F, Greenbaum CJ, et al. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia 2005;48:2206-12. [Crossref] [PubMed]
- Pozzilli P, Buzzetti R. A new expression of diabetes: double diabetes. Trends Endocrinol Metab 2007;18:52-7. [Crossref] [PubMed]
- Porcel Chacón R, Tapia Ceballos L, Ranchal Pérez P. Diabetes latente autoinmune en el joven. Una nueva aportación An Pediatr (Barc) 2015;82:e238-9. [Latent autoimmune diabetes in the young A new case]. [Crossref] [PubMed]
- Olamoyegun MA, Ala OA, Ugwu E. Coexistence of type 1 and type 2 diabetes mellitus: a case report of "double" diabetes in a 17-year-old Nigerian girl. Pan Afr Med J 2020;37:35. [Crossref] [PubMed]
- Arslanian S, Bacha F, Grey M, et al. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care 2018;41:2648-68. [Crossref] [PubMed]
- Tfayli H, Bacha F, Gungor N, et al. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus -positive patients. Diabetes 2009;58:738-44. [Crossref] [PubMed]
- Lee SH, Yu J. Clinical features of childhood diabetes mellitus focusing on latent autoimmune diabetes. Ann Pediatr Endocrinol Metab 2016;21:212-8. [Crossref] [PubMed]
- Xiang Y, Liu B, Yun C, et al. Frequency, clinical features, inflammatory cytokines and genetic background of latent autoimmune diabetes in youth in youth-onset type 2 diabetes: Results from a nationwide, multicentre, clinic-based, cross-sectional study (LADA China). Diabetes Obes Metab 2021;23:1282-91. [Crossref] [PubMed]
- Schloot NC, Pham MN, Hawa MI, et al. Inverse Relationship Between Organ-Specific Autoantibodies and Systemic Immune Mediators in Type 1 Diabetes and Type 2 Diabetes: Action LADA 11. Diabetes Care 2016;39:1932-9. [Crossref] [PubMed]
- Biondi B, Kahaly GJ, Robertson RP. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr Rev 2019;40:789-824. [Crossref] [PubMed]
- Roberts CG, Ladenson PW. Hypothyroidism. Lancet 2004;363:793-803. [Crossref] [PubMed]
- Jonsdottir B, Andersson C, Carlsson A, et al. Thyroid autoimmunity in relation to islet autoantibodies and HLA-DQ genotype in newly diagnosed type 1 diabetes in children and adolescents. Diabetologia 2013;56:1735-42. [Crossref] [PubMed]
- Kahles H, Fain PR, Baker P, et al. Genetics of Autoimmune Thyroiditis in Type 1 Diabetes Reveals a Novel Association With DPB1*0201: Data From the Type 1 Diabetes Genetics Consortium. Diabetes Care 2015;38:S21-8. [Crossref] [PubMed]
- Alyafei F, Soliman A, Alkhalaf F, et al. Prevalence of β-cell antibodies and associated autoimmune diseases in children and adolescents with type 1 diabetes (T1DM) versus type 2 diabetes (T2DM) in Qatar. Acta Biomed 2018;89:32-9. [PubMed]
- Reinehr T, Schober E, Wiegand S, et al. Beta-cell autoantibodies in children with type 2 diabetes mellitus: subgroup or misclassification? Arch Dis Child 2006;91:473-7. [Crossref] [PubMed]
- Libman IM, Sun K, Foley TP, et al. Thyroid autoimmunity in children with features of both type 1 and type 2 diabetes. Pediatr Diabetes 2008;9:266-71. [Crossref] [PubMed]
- Jin P, Huang G, Lin J, et al. High titre of antiglutamic acid decarboxylase autoantibody is a strong predictor of the development of thyroid autoimmunity in patients with type 1 diabetes and latent autoimmune diabetes in adults. Clin Endocrinol (Oxf) 2011;74:587-92. [Crossref] [PubMed]
- Rogowicz-Frontczak A, Pilacinski S, Wyka K, et al. Zinc transporter 8 autoantibodies (ZnT8-ab) are associated with higher prevalence of multiple diabetes-related autoantibodies in adults with type 1 diabetes. Diabetes Res Clin Pract 2018;146:313-20. [Crossref] [PubMed]
- Zampetti S, Capizzi M, Spoletini M, et al. GADA titer-related risk for organ-specific autoimmunity in LADA subjects subdivided according to gender (NIRAD study 6). J Clin Endocrinol Metab 2012;97:3759-65. [Crossref] [PubMed]
- Maioli M, Pes GM, Delitala G, et al. Number of autoantibodies and HLA genotype, more than high titers of glutamic acid decarboxylase autoantibodies, predict insulin dependence in latent autoimmune diabetes of adults. Eur J Endocrinol 2010;163:541-9. [Crossref] [PubMed]
- Toulis K, Tsekmekidou X, Potolidis E, et al. Thyroid Autoimmunity in the Context of Type 2 Diabetes Mellitus: Implications for Vitamin D. Int J Endocrinol 2015;2015:710363. [Crossref] [PubMed]
- Tang X, Yan X, Zhou H, et al. Prevalence and identification of type 1 diabetes in Chinese adults with newly diagnosed diabetes. Diabetes Metab Syndr Obes 2019;12:1527-41. [Crossref] [PubMed]
- Nan X, Li X, Xiang Y, et al. Screening Strategy for Islet Autoantibodies in Diabetes Patients of Different Ages. Diabetes Technol Ther 2022;24:212-9. [Crossref] [PubMed]
- Zhou Z, Xiang Y, Ji L, et al. Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes 2013;62:543-50. [Crossref] [PubMed]
- Xiang Y, Huang G, Zhu Y, et al. Identification of autoimmune type 1 diabetes and multiple organ-specific autoantibodies in adult-onset non-insulin-requiring diabetes in China: A population-based multicentre nationwide survey. Diabetes Obes Metab 2019;21:893-902. [Crossref] [PubMed]
- Rivera-Vega MY, Flint A, Winger DG, et al. Obesity and youth diabetes: distinguishing characteristics between islet cell antibody positive vs. negative patients over time. Pediatr Diabetes 2015;16:375-81. [Crossref] [PubMed]
- Rogowicz-Frontczak A, Zozuliłska-Ziołkiewicz D, Litwinowicz M, et al. Are zinc transporter type 8 antibodies a marker of autoimmune thyroiditis in non-obese adults with new-onset diabetes? Eur J Endocrinol 2014;170:651-8. [Crossref] [PubMed]
- Reghina AD, Florea S, Constantin M, et al. The Impact of Thyroid Autoimmunity on the Clinical and Diabetes Parameters of Patients with Latent Autoimmune Diabetes in Adults. Exp Clin Endocrinol Diabetes 2015;123:543-7. [Crossref] [PubMed]
- Wasserfall C, Nead K, Mathews C, et al. The threshold hypothesis: solving the equation of nurture vs nature in type 1 diabetes. Diabetologia 2011;54:2232-6. [Crossref] [PubMed]
- Klingensmith GJ, Pyle L, Arslanian S, et al. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care 2010;33:1970-5. [Crossref] [PubMed]
- Tfayli H, Bacha F, Gungor N, et al. Islet cell antibody-positive versus -negative phenotypic type 2 diabetes in youth: does the oral glucose tolerance test distinguish between the two? Diabetes Care 2010;33:632-8. [Crossref] [PubMed]


