
Lung transplantation for bronchiolitis obliterans after hematopoietic stem cell transplantation: a retrospective single-center study
Introduction
Hematopoietic stem cell transplantation (HSCT) is a possible radical cure for hematological malignancies and hereditary diseases (1). With the development and progress of technology, more patients gain HSCT opportunities, but complications after transplantation seriously affect its efficacy (2), and concurrent lung disease after HSCT increases the risk of death by nearly 50% (3). Lung injury after HSCT can be divided into infectious and non-infectious types according to the mechanism of occurrence. Among these, non-infectious lung complications are divided into early and late types, and bronchiolitis obliterans (BO) is the most common late non-infectious lung complication. The early clinical manifestations of BO are not obvious and are mainly progressive dyspnea after activity and obstructive respiratory dysfunction suggested by pulmonary function testing. Submucosal bronchiolar fibrosis accompanied by lumen stenosis and occlusion can be shown by histopathological test. Currently, drug therapy is the preferred treatment for BO after HSCT, and lung transplantation (LT) is the only method to cure end-stage disease, although its overall efficacy is rarely reported (4). Therefore, we conducted a single-center retrospective analysis to describe our clinical experience with LT in patients with BO after HSCT. This study is an extension of the existing data, which aimed to provide a reference for the current selection of treatment in patients with post-HSCT end-stage BO. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2517/rc).
Methods
Study design and data collection
We retrospectively analyzed the medical records of six patients with BO after HSCT who received LT in the LT Center of the Affiliated Wuxi People’s Hospital of Nanjing Medical University from 2015 to 2019. The collected information included demographic data, surgery-related conditions, and postoperative follow-up data (Figure 1, Tables 1-3). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (No. KY22009). Written informed consent was obtained from all patients or their agents.
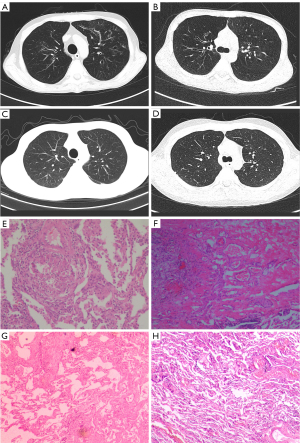
Table 1
| Case | Gender | Age | Time of operation | Protopathy | Age at HSCT | Age at LT | Interval between HSCT and LT (months) | Pulmonary infection before surgery | Extrapulmonary GVHD | Oxygen therapy | pCO2 (mmHg) | FEV1/FVC (%) | FEV1% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 27 | October 2019 | AML | 24 | 27 | 35 | Yes | Yes | Nasal catheter | 60.9 | 36.23 | 13 |
| 2 | Male | 12 | June 2019 | AML | 10 | 12 | 19 | Yes | No | Nasal catheter/non-invasive ventilator | 106.9 | 38.27 | 12.8 |
| 3 | Male | 21 | July 2017 | ALL | 16 | 21 | 66 | Yes | Yes | Nasal catheter | 68 | – | – |
| 4 | Male | 48 | May 2017 | CML | 35 | 48 | 156 | Yes | Yes | Nasal catheter | 57.8 | 29.2 | 15.8 |
| 5 | Female | 23 | July 2016 | ALL | 16 | 23 | 79 | Yes | Yes | Non-invasive ventilator | 83 | – | – |
| 6 | Female | 39 | March 2015 | ALL | 32 | 39 | 75 | No | Yes | Nasal catheter | 49.9 | 81.66 | 25.3 |
AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; CML, chronic myeloid leukemia; HSCT, hematopoietic stem cell transplantation; LT, lung transplantation; GVHD, graft-versus-host disease; pCO2, carbon dioxide partial pressure; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Table 2
| Case | ECMO | Surgery | Left lung cold ischemia time (min) | Right lung cold ischemia time (min) | Blood loss (mL) | Blood transfusion (mL) |
|---|---|---|---|---|---|---|
| 1 | Veno-venous | Double LT | 443 | 600 | 700 | 1,425 |
| 2 | Veno-venous | Double LT + right lung volume reduction | 370 | 470 | 1,000 | 1,175 |
| 3 | Veno-arterial | Right-sided LT | – | 490 | 3,600 | 3,250 |
| 4 | Veno-arterial | Right-sided LT | – | 190 | 600 | 750 |
| 5 | – | Double LT | 443 | 430 | 2,000 | 2,200 |
| 6 | Veno-arterial | Double LT + right lung volume reduction + Nuss technique correction for pectus excavatum | 290 | 168 | 800 | 950 |
ECMO, extracorporeal membrane oxygenation; LT, lung transplantation.
Table 3
| Case | Mechanical ventilation time (days) | Time of ECMO (hours) | Reoperation | FEV1% 3 months after surgery | Immunosuppressive protocol | Time in ICU (days) | Time of discharge (day after surgery) | Follow up (months) | Complication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 22 | No | 47.4 | Ciclosporin + prednisone | 3 | 40 | 28 | Infection |
| 2 | 1 | 21 | No | 83.2 | Tacrolimus + mycophenolate mofetil + corticosteroids | 2 | 14 | 32 | Infection, diabetes mellitus, hyperlipemia |
| 3 | 7 | 7 | No | – | Tacrolimus + corticosteroids | 9 | Die at 9 | – | Infection |
| 4 | 1 | 3 | No | 47.9 | Tacrolimus + mycophenolate mofetil + corticosteroids | 3 | 49 | 57 | Infection |
| 5 | 3 | 0 | Thoracotomy hemostasis | 49.5 | Tacrolimus + mycophenolate mofetil + corticosteroids | 3 | 53 | 67 | Acute rejection, infection |
| 6 | 2 | 5 | No | 61.4 | Tacrolimus + corticosteroids | 3 | 34 | 83 | Infection, osteoporosis |
ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in 1 second; ICU, intensive care unit.
Treatment
All patients were systematically evaluated before surgery, which included blood tests, infection status assessment, lung function assessment, anesthesia assessment, and function assessment of other organs. According to the assessment results and the 2006 guidelines of the International Society for Heart and Lung Transplantation (ISHLT) (5), multidisciplinary experts then undertook a preoperative discussion to determine whether patients should be accepted for LT.
Organs for transplants were donated by volunteers, and our center did not receive lungs from executed prisoners (6). Five donors were assessed as brain death and another was assessed as cardiac death. The ABO blood groups of the donor and recipient were the same before surgery. Preoperative chest radiograph or computed tomography (CT) showed no lung infection or other lung disease, and the oxygenation index was over 300 mmHg.
Single or double LT was performed according to donor lung and patient condition. In general, when the recipient was frail and elderly or bled more than 3,000 mL after unilateral LT, we considered only doing single LT. The need for intraoperative extracorporeal membrane oxygenation (ECMO) support was determined by the oxygenation index, pulmonary artery pressure, and hemodynamic stability after 15 minutes of single-lung ventilation. ECMO support was not considered if hemodynamics were stable and percutaneous oxygen saturation remained above 90%. If hemodynamics were stable and percutaneous oxygen saturation continued to be below 90%, veno-venous ECMO was used, and if hemodynamics were unstable and percutaneous oxygen saturation persisted below 90%, veno-arterial ECMO was used. All patients were transferred to the intensive care unit (ICU) after surgery, where they received mechanical ventilation and antibiotic treatment to prevent infection. Anti-rejection therapies include tacrolimus, ciclosporin, mycophenolate mofetil, and corticosteroids, and imaging, bronchoscopy, and blood gas analysis were performed regularly. All patients were regularly followed up after discharge, which in the first year, was performed every 3 months. Over the next 2 years, patients were assessed every 6 months, and after 3 years, the frequency was once annually. The assessment contents included lung and other organ functions, patients’ immune levels, and infection status.
Statistical analysis
All data were descriptive statistics with the format of mean ± standard deviation, analyzed by SPSS version 26.0.
Results
From March 2015 to November 2019, six patients with BO after HSCT received LT at our center. There were four males and four females, and their average age was 28±13 years. Each had received HSCT for blood disorders at an average age of 22±10 years, including acute lymphocytic leukemia (ALL), acute myeloid leukemia (AML), and chronic myeloid leukemia (CML). At a mean 72±48 months after HSCT, patients were transferred by local hospitals to our center for LT evaluation as they developed end-stage BO. Prior to this, all had received standard drug therapy. The chief complaint of all six patients was dyspnea, among which five had pulmonary infection and five had extrapulmonary graft-versus-host disease (GVHD). All patients developed hypercapnia with an average carbon dioxide partial pressure (pCO2) of 71.1±20.8 mmHg and were treated with oxygen. Case 5 used a non-invasive ventilator, case 2 alternately used a nasal catheter and non-invasive ventilator, and the remaining patients only used a nasal catheter. Only four patients received a pulmonary function test because case 3 and case 5 could not tolerate it. The actual value of forced expiratory volume in 1 second (FEV1) was 16.7%±5.9% of the predicted value, and the average ratio of FEV1 to forced vital capacity (FVC) was 46.34%±23.87%. Preoperative chest CT of case 1 and case 2 indicated dilatation of some bronchi in both lungs with surrounding patchy and nodular fuzzy shadows (Figure 1A,1B). Detailed preoperative data are shown in Table 1.
After assessment, four patients adopted sequential bilateral LT and two adopted right-sided LT. Case 3 and case 4 did not adopted left-sided LT respectively because of persistent atrial fibrillation and excessive bleeding. In case 2 and case 6, the volume of the right donor lungs was reduced by cutting the middle lobe because they were of larger size than the recipient chest. In addition, the lingual segment of the left donor lung was cut in case 6, who also underwent simultaneous Nuss technique correction for pectus excavatum. The mean cold ischemia time of the left lung was 416±115 min and of the right lung 391±174 min. During operation, the mean blood loss was 1,450±1,169 mL and the mean blood transfusion was 1,625±942 mL. Due to hemodynamic instability, five patients adopted intraoperative assistance of ECMO, with three receiving veno-arterial ECMO and two veno-venous ECMO. Intraoperative data are shown in Table 2. Pathological examination showed changes of BO (Figure 1E-1H).
After operation, all patients were transferred to ICU for treatment where the average mechanical ventilation duration was 3±2 days, the average ECMO duration was 10±9 h, and the time in ICU was 4±3 days. Postoperative chest CT of case 1 and case 2 showed good lung dilation after bilateral LT (Figure 1C,1D), while case 5 underwent reoperation due to hemothorax on the first postoperative day and developed acute rejection on the fourth postoperative day, which was improved after treatment. With the exception of case 3 who died of septic shock on the 9th day, five patients were discharged 38±15 days after surgery. At the time of follow-up, the 5 remaining patients were well and out of the hospital, and their quality of life was much better than before operation. The mean follow-up time to date is 53±23 months. After discharge, four patients took tacrolimus and prednisone to suppress immunity, three of whom also took mycophenolate mofetil, and another took tacrolimus and cyclosporine. The actual value of FEV1 at 3 months postoperatively accounted for 57.9%±15.3% of the predicted value, and no patients had recurrence of BO. After surgery, all patients developed different degrees of infection which improved after drug treatment, while case 2 developed diabetes mellitus and hyperlipidemia and case 6 developed osteoporosis. Detailed postoperative data are shown in Table 3.
Discussion
BO is one of the most common late non-infectious pulmonary complications after HSCT, which seriously affects the long-term survival and quality of life of patients. First described in 1982 by Roca et al. (7), the disease is a progressive airflow obstruction syndrome that primarily involves small airways and is independent of acute GVHDs, infection, and other confounders (8). The prevalence of BO after HSCT is only 2–3% (9), the mortality is high, and the prognosis is poor. The case fatality rate can be as high as 80% (10). BO usually occurs 1 year after transplantation, and due to the insidious onset and non-specific clinical symptoms and signs, early diagnosis and treatment remains challenging. Dry cough and dyspnea after activity are the main early symptoms, and in addition to pulmonary signs, almost all patients have symptoms of chronic GVHD such as dry skin, pigmentation, dry eyes, photophobia, cholestasis, and abnormal liver function. All six patients in this study had dyspnea, and five had extrapulmonary GVHD. According to the consensus of BO diagnostic criteria formulated by the National Institutes of Health (NIH) in 2005 and the revised version in 2014 (11,12), all six patients were diagnosed with post-HSCT BO in local hospitals. The NIH established symptom score and lung function score is based on the severity of the disease (12) which allocates 0 as asymptomatic, 1 as asthma when climbing stairs, 2 as asthma when walking on flat ground, and 3 as asthma at rest or requiring oxygen treatment. In lung function score, 0 is FEV1 ≥80%, 1 is 60%≤FEV1 ≤9%, 2 is 40%≤ FEV1 ≤59%, and 3 is FEV1 ≤39%. Our patients scored 3 on either score, which indicated the disease had progressed to its terminal stage. According to a previous study (13), the risk of non-recurrent death in BO patients with an NIH symptom score of 3 is 5.6 times higher than in those with an NIH symptom score of 0.
At present, there is no unified treatment plan for BO at home or abroad, and most early treatment experience comes from small-scale uncontrolled clinical trials and expert experience. The treatment of BO includes immunosuppressive therapy, extracorporeal photochemotherapy, and surgical treatment. Among these, immunosuppressive therapy is considered as the main treatment for BO after HSCT as current studies indicate its pathogenesis is related to a variety of complex immune responses (8,14). At local hospitals, our patients received standard immunosuppressive therapy for a long duration, but their lung function continued to deteriorate and eventually the disease progressed to the terminal stage. LT is recommended for these patients who have progressive deterioration of lung function, no response to various drug treatments, and no surgical contraindications (15).
LT is the only method which may cure patients with end-stage BO, but the overall long-term prognosis remains poor. Vogl et al. reported seven cases of BO after HSCT whose median survival time after LT was only 24 months (16), with most developing chronic lung GVHD or recurrent BO within 5 years. In a systematic review (17), 81% of 84 patients undergoing LT after HSCT developed BO or pulmonary fibrosis, with a 36-month survival rate of 76% and a probable 2- and 3-year survival rate of 88% and 79%, respectively. Thus, it can be seen the long-term prognosis of post-HSCT BO patients who receive LT is related to the recurrence of BO. In addition, there are concerns that LT may lead to the recurrence of malignant diseases treated with HSCT. The ISHLT does not recommend LT for patients with malignant disease in the first 2 years after HSCT (5). In this study, the interval between HSCT and LT was 72±48 months, with only one case less than 2 years. All patients recovered well, with no recurrence of BO or blood disease, except one patient who died from infection. In conclusion, LT may be a treatment worthy of consideration in patients with post-HSCT end-stage BO because it can improve lung function, quality of life and prolong survival of these selected patients. However, all studies at this stage have some limitation because of the small number of cases included (4,16,18), which is due to the low prevalence of BO after HSCT, and large-scale prospective clinical studies are necessary to determine clinical efficacy. Our study aims to elucidate the clinical experience of our single center and provide a reference for the current selection of treatment.
Acknowledgments
Funding: This work was supported by the High-Level Talent Training Project of Wuxi Taihu Talent Plan (No. BJ2020004) and the Key Laboratory of Pathogen Biology of Jiangsu Province (No. KLPBJP-K202002).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2517/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2517/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2517/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (No. KY22009). Written informed consent was obtained from all patients or their agents.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol 2012;12:443-58. [Crossref] [PubMed]
- Nakaseko C, Ozawa S, Sakaida E, et al. Incidence, risk factors and outcomes of bronchiolitis obliterans after allogeneic stem cell transplantation. Int J Hematol 2011;93:375-82. [Crossref] [PubMed]
- Jayasuriya G, Lin B, Keogh SJ, et al. Pulmonary Complications of Malignancies and Blood and Marrow Transplantation. In: Koumbourlis AC, Nevin MA, editors. Pulmonary Complications of Non-Pulmonary Pediatric Disorders. Cham: Springer International Publishing, 2018:51-77.
- Jung HS, Lee JG, Yu WS, et al. Early outcomes of lung transplantation for bronchiolitis obliterans syndrome after allogeneic haematopoietic stem cell transplantation: a single-centre experience. Interact Cardiovasc Thorac Surg 2016;23:914-8. [Crossref] [PubMed]
- Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55. [Crossref] [PubMed]
- Wu B, Hu C, Chen W, et al. China lung transplantation developing: past, present and future. Ann Transl Med 2020;8:41. [Crossref] [PubMed]
- Roca J, Grañeña A, Rodriguez-Roisin R, et al. Fatal airway disease in an adult with chronic graft-versus-host disease. Thorax 1982;37:77-8. [Crossref] [PubMed]
- Grønningsæter IS, Tsykunova G, Lilleeng K, et al. Bronchiolitis obliterans syndrome in adults after allogeneic stem cell transplantation-pathophysiology, diagnostics and treatment. Expert Rev Clin Immunol 2017;13:553-69. [Crossref] [PubMed]
- Chien JW, Duncan S, Williams KM, et al. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2010;16:S106-14. [Crossref] [PubMed]
- Vieira AG, Funke VA, Nunes EC, et al. Bronchiolitis obliterans in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant 2014;49:812-7. [Crossref] [PubMed]
- Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945-56. [Crossref] [PubMed]
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21:389-401.e1. [Crossref] [PubMed]
- Gazourian L, Rogers AJ, Ibanga R, et al. Factors associated with bronchiolitis obliterans syndrome and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Am J Hematol 2014;89:404-9. [Crossref] [PubMed]
- Kuroi T, Fujii N, Ichimura K, et al. Characterization of localized macrophages in bronchiolitis obliterans after allogeneic hematopoietic cell transplantation. Int J Hematol 2021;114:701-8. [Crossref] [PubMed]
- Gao F, Chen J, Wei D, et al. Lung transplantation for bronchiolitis obliterans syndrome after allogenic hematopoietic stem cell transplantation. Front Med 2018;12:224-8. [Crossref] [PubMed]
- Vogl UM, Nagayama K, Bojic M, et al. Lung transplantation for bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation: a single-center experience. Transplantation 2013;95:623-8. [Crossref] [PubMed]
- Soubani AO, Kingah P, Alshabani K, et al. Lung transplantation following hematopoietic stem cell transplantation: report of two cases and systematic review of literature. Clin Transplant 2014;28:776-82. [Crossref] [PubMed]
- Suzuki J, Kasai H, Terada J, et al. Bronchiolitis obliterans after stem cell transplantation for hematologic malignancies rescued by lung transplantation: A report of two cases. Respir Investig 2021;59:559-63. [Crossref] [PubMed]
(English Language Editor: B. Draper)


