
Percutaneous full endoscopic C1 laminectomy for developmental atlantal stenosis with myelopathy: a case report of three cases and review of the literature
Introduction
Despite its low prevalence, atlas deformity associated with spinal cord compression constitutes a potentially devastating condition, which may lead to quadriplegia and even death (1,2). In 1989, Sawada et al. (3) first reported the use of C1 laminectomy (C1L) for a case of canal stenosis at the level of the atlas. Subsequently, in 1994, seven cases of cervical myelopathy caused by aplasia of the posterior arch of the atlas (PAA) were reported by Currarino (4). Shah et al. (5) and Bhattacharjee et al. (6) also described similar cases of abnormal development of the C1 posterior arch which led to stenosis of the spinal canal. However, almost all of the relevant articles were case reports. Recently, Wang et al. (7) found the pathophysiological mechanism of developmental atlantal stenosis with myelopathy (DASM) was complex and multifactorial, and the clinical symptoms were diverse in a series of 15 cases.
Surgery is the most effective treatment for DASM, and can significantly relieve spinal cord compression, ameliorate myelopathy symptoms, and improve spinal cord function. At present, the most common surgical method for DASM is C1L with or without atlantoaxial fixation and fusion (8-11). Although this open surgery can improve neurological symptoms, it requires wide surgical exposure that may introduce remarkable operative trauma, and the operation risk is relatively high, especially in elderly populations with comorbidities.
Minimally invasive spine surgery (MISS) offers alternative treatment options with advantages. Endoscope systems expand the technical capabilities of surgeons, which allows safer and more effective therapeutics for difficult and complicated diseases (12,13). In the past decade, endoscopic spine surgery has been widely used for lumbar, thoracic, and cervical degenerative disease (14,15). Here we present a novel approach for the treatment of DASM by percutaneous full endoscopic C1 laminectomy (PFEC1L) to take over the disadvantage of C1L, such as soft tissue damage and massive hemorrhage as an open operation. To the best of our knowledge, it has not been previously reported. In addition, we review and summarize the relevant literature and discuss the etiology, pathophysiology, and diagnostic and treatment strategies for DASM. We present the following article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2282/rc).
Case presentation
Ethics
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
A 66-year-old male patient presented to our hospital with complaints of neck pain and numbness and weakness in the limbs for more than 7 months. His symptoms were aggravated in the past 2 months and included walking instability and difficulty grasping and holding objects. Neurological signs including Hoffman, Babinski and ankle clonus were positive, and muscle strength of the upper and lower extremities was grade IV. Magnetic resonance imaging (MRI) demonstrated spinal cord compression, as well as high signal change on T2-weighted imaging (T2WI) at the level of the atlas (Figure 1). A preoperative sagittal computed tomography (CT) scan showed a 7.5-mm diameter of the C1 space available for the cord (SAC), and preoperative flexion and extension cervical radiographs revealed good atlantoaxial stability. A cervical CT angiography (CTA) examination was performed to specifically assess the position of the vertebral artery (VA) and estimate the distance between the VA and PAA. While the patient was treated conservatively in the clinic for 8 weeks before being hospitalized, the symptoms were not notably relieved or even aggravated. The preoperative modified Japanese Orthopaedic Association (mJOA) score was 8 points (8/17), and the visual analogue scale (VAS) was 5 points (5/10). The patient finally underwent robot-assisted PFEC1L after excluding contraindications on December 2018.
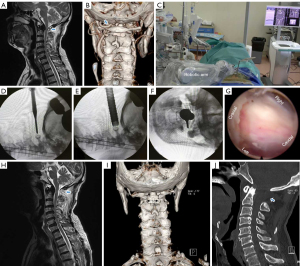
The operative time was 65 minutes and blood loss was minimal. No drainage, painkillers, or antibiotics were needed postoperatively, and the patient was discharged on the fifth postoperative day without problem. The 3-month postoperative mJOA score was 11 points (11/17), and 2-year postoperative was 14 points (14/17), at which time the patient reported slight limbs numbness without weakness or pain, and his mobility has basically returned to normal at 30-month postoperative follow-up. Postoperative MRI showed significant improvement of spinal cord compression and the three-dimensional CT showed the removed section of the PAA was about 1.9 cm (Figure 1).
Case 2
An 83-year-old women experienced numbness in the extremities for 2 years. She had slight asthenia in the right upper limb, and the clinical manifestations had progressed in the past 6 months to where she could not walk without difficulty. The patient had a history of coronary heart disease, hypertension, type 2 diabetes mellitus, osteoporosis, and hyperlipidemia. Neurological examination of the sensory system revealed disturbance of deep sensation, which manifested as hyperreflexia of the patellar and Achilles tendons and a positive Romberg’s test. The tongue and palate moved normally without atrophy or fasciculation, and there was neither atrophy nor wasting of the sternocleidomastoid muscles, although slight muscular wasting was observed in the right arms and forearms. Preoperative CT scan of the cervical spine revealed a markedly narrow canal at the level of the atlas, where the SAC was 7.0-mm. Preoperative MRI showed the deformed PAA compressing the spinal cord, and spinal cord compression was also observed at the location of C5–6 (Figure 2). Preoperative X-ray did not reveal obvious atlanto-axial subluxation or instability, and the mJOA score was 8 points (8/17) and VAS score 3 points (3/10). The patient was treated surgically by robot-assisted PFEC1L and percutaneous full endoscopic C5/6 decompression on October 2019, with a total operative time of 118 min, of which 62 min was for PFEC1L. The patient’s neck pain was alleviated at the early postoperative stage, and she was discharged 3 days after surgery. At 2-year after surgery, her weakness and numbness of the extremities were alleviated, the motor examination showed improvement, and the postoperative mJOA score was 13 points (13/17) and VAS score 1 point (1/10). Postoperative MRI revealed spinal cord decompression was sufficiently attained, and CT scan demonstrated the PAA was partial removed (Figure 2).
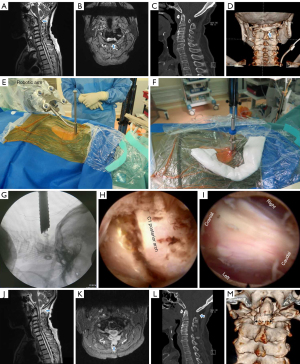
Case 3
A 52-year-old female with bilateral hand numbness and left lower limb weakness for 7 years complained of neck pain at the upper cervical spine level exacerbated with extension and rotation to either side. The patient had a history of severe anaemia and osteopenia. Physical examination revealed a short neck, hyperreflexic bilateral triceps, brachioradialis, patellar tendon, and Achilles tendon reflexes, and positive Romberg, Hoffman, and Babinski signs. The left lower limbs, including hip flexors, knee extensors, ankle dorsiflexors, and ankle plantar flexors, showed grade IV strength. Initial MRI showed spinal cord narrowing thickness and heterogeneous signal at the level of C1, and the spinal cord compression due to abnormal PAA was particularly severe on the left side. A preoperative CT scan revealed a markedly narrow canal at the level of the atlas, and the SAC was 7.0 mm (Figure 3), and the mJOA scored 9 points (9/17) and VAS 4 points (4/10). Conservative treatment was commenced and continued for 3 months with virtually no improvement, and the patient accepted PFEC1L in July 2020.
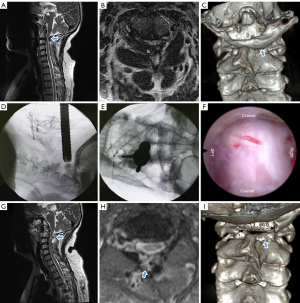
The operation took 70 minutes to complete, after which she was able to ambulate on the 1st postoperative day and was discharged well 4 days post-operation. The patients’ symptoms were comparably improved significantly 1-year after surgery, and the mJOA was 14 points (14/17) and VAS score 1 point (1/10).
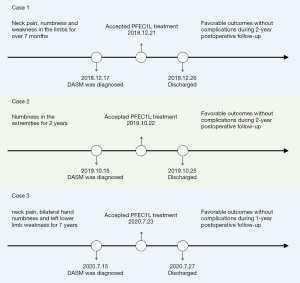
The whole process of diagnosis and treatment of DASM by PFEC1L in three cases was outlined in Figure 4.
Method of PFEC1L
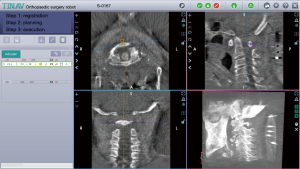
The procedure was performed under general anesthesia with patients placed in the prone position. To begin with, planning of the working-channel endoscope trajectories was performed on the robotic workstation (Figure 5). With the help of the robot, a Kirschner wire was accurately established to the posterior tubercle of the atlas, then the dilating tube and working channel were rapidly placed along the wire. All subsequent steps were performed using the endoscope and non-bacteriostatic saline irrigation, including removal of the PAA and decompression of the spinal cord.
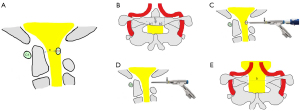
Under the endoscope, soft tissue around the PAA was cleaned until its upper and lower boundaries were clearly distinguishable, with the posterior tubercle taken as the center and expanded to the left and right sides by 1 cm each. The external cortical bone and internal cancellous bone were then removed using a high-speed drill, and the internal cortical bone was temporarily preserved. The internal cortical bone was carefully removed using a Kerrison rongeur, followed by exposure of the intact spinal cord by careful removal of the dorsal ligament. After adequate decompression of the spinal cord was achieved, a bipolar RF device was used for hemostasis, the endoscope and working tube were exited, and the wound was closed using surgical glue.
Literature search
PubMed, Ovid MEDLINE, and EMBASE were searched for relevant articles. The search terms were: [(“C1 stenosis” or “atlas stenosis” or “atlantal stenosis” or “atlantoaxial stenosis” or “atlanto-axial stenosis”) AND (“C1 hypoplasia” or “C1 malformation” or “atlas hypoplasia” or “atlas malformation”)]. All articles of any study design discussing DASM were considered for inclusion, while experimental or animal studies and non-English language studies were excluded. After an initial screen of abstracts and article titles, full text articles of all potential studies were obtained.
A total of 232 studies were identified from the initial search, then 27 duplicates, non-English, and animal studies were removed. Titles and abstracts of the 205 remaining studies were screened according to the pre-defined inclusion criteria, and 161 studies were excluded, leaving 44 articles for critical review and consolidation for this literature review. Figure 6 shows the flowchart.
Discussion
Etiology and pathophysiology of DASM
The appearance of DASM is highly unusual in adults, and its pathogenesis remains poorly understood. The development of atlantal stenosis involves several factors, among which a genetic cause is one of the most important (16). The occurrence of atlas developmental malformation is more common in patients with Chiari malformation, Down syndrome, Klippel-Feil syndrome, Noonan syndrome, Williams syndrome, diffuse idiopathic skeletal hyperostosis (DISH), atlanto-axial instability, and atlantoaxial subluxation with or without os odontoideum (4,5,17-24). Stenosis secondary of C1 without trauma and atlantoaxial instability is a possible pathophysiologic factor, which may be secondary to atlas hypoplasia, ossification of the posterior atlantoaxial membrane (PAAM) (20), or ossification of the transverse atlantal ligament (OTAL) (25) that lead to consistent space reduction of the spinal cord. The developmentally narrowed spinal canal of the atlas in minors is generally displayed as the inturning of a bifid posterior arch, partial atlas agenesis, or absence of the PAA (4,26-29). However, in adults, the main deformities are usually an asymmetric structure, abnormal bone hyperplasia, and inborn spinal canal stenosis with complete PAA (30,31). On rare occasions, it can also show dysmorphic features with failure of fusion of the two hemi-arches with a midline cleft as reported by Shah et al. (5) in a 44-year-old female. We speculate DASM may be related to genes and age, and the deformity develops incrementally with age. According to the limited existing literature available, DASM is more commonly found in middle-aged and elderly individuals over 50 years old (8).
Subtypes of DASM
Depending on the etiology, atlantal stenosis can be classified as developmental or secondary (6,32,33). DASM is a congenital developmental deformity of the atlas resulting in reduction of the spinal canal diameter and spinal cord compression. Atlas developmental deformities mainly manifest at three sites, including the anterior arch, posterior arch, and atlantoaxial lateral masses. Among these, the prevalence of posterior arch malformation is the highest (7) and can lead to a congenitally small spinal canal and subsequent myelopathy. It was reported that 4% of 1,613 autopsies showed posterior arch deformity of the atlas, which can be divided into two types: median fissure or hypoplasia (34,35). In another study, hypoplasia of the PAA was divided into spondyloepiphyseal dysplasia congenita and an idiopathic type (36).
Currarino et al. (4) divided congenital anomalies of the posterior arch into five types according to different morphologies of the PAA, including (I) failure of posterior midline fusion of the two hemiarches, (II) unilateral clefts, (III) bilateral clefts, (IV) absence of the posterior arch with persistent posterior tubercle, and (V) absence of the entire arch including the posterior tubercle, which is a classification method mainly based on abnormal morphological changes of the PAA in minors. According to the morphological analysis of CT image data, Wang et al. (7) divided DASM into four subgroups, including (I) small size atlas, (II) hypertrophy of PAA, (III) incurved of PAA, and (IV) hypertrophy of the odontoid. Among these, type I is relatively common, type II and III are relatively rare, and type IV are the least common.
Clinical manifestations of DASM
While anomaly of the PAA is an easily overlooked cause of symptomatic cervical myelopathy, its clinical symptoms can be very severe. The symptoms of DASM are complex and diverse, including occipital neuralgia, occipitocervical pain, paresthesia and weakness of limbs, gait disturbance, poor fine motor skills and difficulties in holding or grasping, urinary incontinence, ataxia, and apnea (5-8,10,21,31,33,37). Certain cervical positions can induce or exacerbate these symptoms, and movement restrictions in the upper cervical spine can be observed in some patients. Furthermore, breathing difficulty is observed in patients with severe worsening of the condition (6).
Imaging characteristics of DASM
Yamahata et al. (38) measured the internal anterior posterior diameter (IAPD) at the C1 level of 213 cases by CT scan, and the results showed the IAPD was 30.7 mm in males and 28.7 mm in females. Musha et al. (8) found the IAPD was 34.4 mm in Japanese females and 37.1 mm in males, and that atlantal stenosis was confirmed when the IAPD less than 29.4 mm in females and 30.5 mm in males. In another study of 543 cadaveric cervical spine specimens (39), the mean C1 inner sagittal diameter was 30.8 mm, and a value below 26.1 mm was defined as atlas dysplasia. Although malformations of the spinal canal of the atlas can be defined by the IAPD, the SAC in relation to the dens and the C1 inner sagittal diameter are more important and sensitive indicators to show the useful activity space of the spinal cord.
In accordance with the results of Hinck et al. (40), the normal range of the SAC is 15 to 20 mm. It is generally assumed that atlas dysplasia can be defined when the SAC is less than 14 mm (41) and clinical signs and symptoms will develop when it is less than 10 mm (42). Consistent with the results of the above studies, the SAC of the cases in the present study was 7 to 7.5 mm. It should be emphasized that MRI provides an important basis for the preoperative diagnosis and treatment strategy, and the antero-posterior diameter of the spinal cord on the sagittal section measured by T1-weighted MRI would be even smaller than that measured by CT. In addition, the T2WI can clearly show the cord compression and signal change of the spinal cord.
Diagnostic criteria of DASM
At present, there is no literature to standardize the diagnostic criteria of DASM. We contemplate that the diagnosis of DASM is mainly based on cervical CT scan, MRI examinations, and clinical symptoms, in which case the following aspects must be taken into account: (I) the SAC is less than 10 mm; (II) MRI reveals spinal cord compression and signal changes, or even spinal cord edema; (cervical CT myelography is useful to show the location and severity of spinal cord compression if the patient is unable to obtain an MRI image); (III) clinical manifestations of spinal cord injury, which are primarily referable to a loss of sensory or motor function in limbs with positive pyramidal sign and pain with limitation of movement in the cervical spine; (IV) cases in which symptoms and signs cannot be explained by intraspinal compression in other locations such as the lower cervical and thoracic spine, or other central nervous system disorders.
Surgical options and outcomes of DASM
The principles of treatment for DASM are to prevent sudden death from neurological compromise and improve neurological status and the quality of life. Wang et al. (7) recommended the appropriate surgical treatment for development spinal canal stenosis at atlas should be selected according to the pathologies, clinical manifestations, and imaging findings. They devised four primary surgical modalities, including posterior arch osteotomy, posterior arch resection and replantation, occipital cervical fixation and fusion, and odontoid reduction and atlantoaxial fixation by a transoral approach. Kim et al. (43) introduced a technique of C1 double-door laminoplasty augmented with an allograft spacer and a titanium miniplate, which allows bone grafting, decompression, and fusion to be performed without disruption of the C1 posterior arch. However, the use of internal fixation increases the cost and the risk of implant loosening and requires sacrificing the function of craniocervical and cervical motion. Hott et al. (44) noted that posterior C1–2 fusion can lead to more than a 50% rotational restriction of the upper cervical spine.
Indeed, an increasing number of reports have suggested a favorable outcome could be achieved by decompression alone for DASM without C1–2 instability and atlantoaxial dislocation. Kawabori et al. (18) reported a case of unique C1 posterior tubercle impingement and myelopathy caused by DISH, in which the patient underwent laminectomy from C1–3, and the myelocompression and myelopathy were improved after operative intervention. Overall, resection of the PAA without fixation and fusion is the most common surgical procedure according to the published literature.
It is worth mentioning that DASM in elderly patients often complicates with subaxial cervical stenosis, which present difficulties for precision diagnosis and a surgical plan. Although major surgeries, such as laminoplasty or laminectomy from C1 to C7, can complete spinal cord decompression in a single procedure, the surgical trauma and risk of complications should not be underestimated. As indicated by Yamahata et al. (45) the pathophysiology of spinal canal stenosis should be considered separately at the C1 and the subaxial cervical spine, and staged surgery is our coping strategy for this kind of situation if the duration of surgery is longer than 3 hours. The segment with the most severe spinal cord compression should be treated firstly by minimally invasive techniques, and a second surgery should be performed at least 6 months after the first if required. The benefit of staged surgery is that it can decrease anesthesia and operating time and reduce the risk of complications. In this study, all three patients obtained satisfactory results through a single operation, avoiding a second.
Although conventional C1L is the most common procedure for DASM, it has the disadvantage of soft tissue damage and massive hemorrhage as an open operation. Notani et al. (35) reported a case of dynamic paraspinal muscle impingement after C1L which led to postoperative neurological deficits and eventually required a revision surgery of occiput to C3 fixation with instrumentation. In addition, Yeom et al. (46) reported extensive dissection and retraction for articular fusion may cause postoperative occipital neuralgia.
Feasibility of endoscopic surgery for DASM
The surgical plan should also depend on the patient’s comorbid conditions and bone quality. For frail elderly individuals comorbid with cardiovascular and cerebrovascular diseases, diabetes, and severe osteopetrosis, MIS can promote rapid recovery after surgery (47,48). In contrast to conventional open procedures, endoscopic spine surgery does not require extensive soft-tissue stripping, resulting in minimal occurrence of postoperative muscle impingement (49). It also has the advantages of fewer incisions, less pain, less bleeding, and less scarring (49,50).
In the cases reported in the present study, full endoscopic spine surgery was confirmed to be an effective method for treating DASM. However, the following details need to be brought to attention. The anatomical position of the atlas is deep and adjacent to several delicate vital organs such as the spinal cord, VA, and nerve roots. Therefore, how to establish the working channel on the surface of the PAA without bringing complications is the first key factor to consider. For DASM, the risk of VA injury is relatively high due to anatomical variations. Careful review of pre-procedural radiological imaging should be performed by the surgeon before planning the procedure, and the CTA superimposed on a CT three-dimensional reconstruction is especially helpful to estimating abnormalities of the VA. The posterior tubercle is an important anatomical landmark which can be taken as the starting point from where to remove the PAA to both sides until there is no compression on the spinal cord (Figure 7). According to an anatomical study from Cacciola et al. (51) the distance between the most medial edge of the VA groove over the PAA and the posterior tubercle of atlas vertebrae is about 14.6 mm. Consequently, removal of the PAA on each side of the posterior tubercle should not be extended beyond this span. Garrido et al. (52) also advocated that the posterior aspect of the PAA should not be exposed over 15 mm. In our experience, resection about 9 to 10 mm of the PAA from the midline is substantial enough to provide adequate decompression.
Advantages of robot assistance
Robot-assisted orthopaedic surgery has been proven successful for accurate surgical planning in pedicle screw placement and spine tumor surgery (53-56), and recent literature suggests incorporating robotic guidance in MISS provides a high degree of accuracy and reduction of radiation exposure (53,57,58). The challenging anatomy of the C1 mini lamina demands highly precise surgical maneuvers (59,60), especially for the percutaneous full endoscopic technique. With the help of robotic precise planning and navigation, the Kirschner wire and working sleeve can be quickly and safely anchored at the midpoint of the posterior tubercle of the atlas, and the anchor point left by the wire can also be used as an important reference for recognizing endoscopic anatomical boundaries.
Indications and limitations of PFEC1L
It is mainly applicable to the stenosis of the atlas canal caused by the posterior arch deformity of the atlas in a single segment. It is not suitable for the lesions of multiple segments, and the instability or dislocation of the atlas. For PFEC1L operation, surgeons are required to have skilled experience in cervical endoscopy, and carefully identify the variant posterior arch of atlas to prevent spinal cord injury.
Strengths and limitations of this study
This study is the first to present the new surgery method of PFEC1L for the treatment of DASM and summarizes the relevant literature to discuss the pathological mechanism, diagnosis, and treatment strategy for this condition (Table 1). However, the limitation of our study is its low number of cases without a control group.
Table 1
| No. | References | Sex | Age (years) | MSCD (mm) | Symptoms | Therapy | Using fixation | Results |
|---|---|---|---|---|---|---|---|---|
| 1 | Tokiyoshi et al. (11) | M | 55 | 8 | Gait disturbance, numbness of the fingers, toes and the proximal parts of the limbs. Left shoulder pain after flexing the neck | C1L and dorsal opening of the foramen magnum | No | Normal gait and no sensory disturbance |
| 2 | Phan et al. (9) | M | 80 | 8 | Bilateral hand numbness and leg stiffness, urinary frequency | C1L and removal of the superior part of the lamina of C2 | No | Postoperative superficial wound infection. However, the symptoms were partially improved |
| 3 | Phan et al. (9) | M | 75 | 7 | Weakness, numbness, and stiffness in extremities. Walking and initiating micturition difficulty | C1L | No | The symptoms improved remarkably |
| 4 | Hsu et al. (10) | M | 38 | 6.23 | Tingling sensations in the abdomen and perineum when flexing the neck, and numbness of both hands | C1L with duraplasty | No | The numbness and the abnormal tingling sensations improved |
| 5 | Kasliwal et al. (30) | F | 26 | – | Posterior cervical headaches with tingling and numbness involving right arm, trunk, and leg | Hemilaminectomy of the atlas preserving the C1/C2 joint | No | Patient had an uneventful postoperative course |
| 6 | Musha et al. (8) | F | 50 | 8 | Gait disturbance, numbness in the bilateral upper and lower limbs | C1L and occipito-cervical fusion (Occ-C2–3) | Yes | Numbness disappeared, loss of manual dexterity and spastic gait alleviated |
| 7 | Musha et al. (8) | M | 75 | 9.5 | Occipital and neck pain, numbness of both hands and feet, weakness of both legs and gait disturbance | C1L and Occ-C2–3 fusion, and expansive laminoplasty at C4–5 | Yes | Motor function improved; urinary incontinence disappeared. sensory abnormalities significantly relieved |
| 8 | Kawabori et al. (18) | M | 75 | – | Numbness in both lower extremities, gait disturbance, disturbed precise motion of the hands, and urinary disturbance | Prophylactic posterior decompression between C1 and C3 | No | Finger motion became smooth and the urinary disturbance disappeared, but dysesthesia still evident |
| 9 | Iki et al. (20) | M | 81 | 9 | Numbness and weakness of extremities and gait disturbance, needing a wheelchair | Laminectomy of C1 and partial C2 | No | Neurological status improved 1 year postoperatively |
| 10 | Yunoki (37) | M | 74 | 9 | Gait disturbance, and clumsy hands | C1L | No | An uneventful postoperative course |
| 11 | Tsuruta et al. (59) | F | 79 | 8 | Occipitalgia, gait impaired, requiring a cane | C1L | No | Occipitalgia disappeared, hemiparesis improved, able to walk without a cane |
| 12 | Bokhari et al. (60) | F | 68 | – | Walking and gait unbalanced, quadriparesis, weakness on the right side, hypoesthesia of the upper extremities | C1L | No | Able to walk with mild assistance 6 months postoperatively |
| 13 | Tang et al. (31) | F | 58 | 5.5 | Neck pain and limitation of neck rotation. numbness of all four limbs and disturbance of gait | C1L | No | Significant improvement in limb numbness and gait disturbance |
| 14 | Sawada et al. (3) | M | 38 | 7 | Right forearm and weakness of the right upper and both lower limbs, walking difficulty | C1L | No | Weakness and spasticity of the extremities were alleviated |
| 15 | Shah et al. (5) | F | 44 | 6.36 | Persistent neck pain for 6 weeks, which had gradually progressed to radiate into the right half of the body, associated with tingling and numbness in the right half of body | En-bloc wide excision of anomalous arch with occipito-cervical fusion | Yes | Excellent clinical outcome without any obvious complaints or disability 2 years later |
DASM, developmental atlantal stenosis with myelopathy; MSCD, mid-sagittal spinal canal diameter; C1L, C1 laminectomy; SAC, space available for the cord.
Conclusions
In summary, DASM is rare but potentially dangerous. Its diagnosis is made mainly based on clinical manifestations combined with radiological imaging examinations, especially CT scan and MRI. While C1L is the most common surgical method, PFEC1L is a new therapeutic option that has the advantage of being minimally-invasive, and the robot-assisted system could improve the accuracy of the working-channel placement.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 82004385 to YPL, No. 82174396 to BC); the Natural Science Foundation of Guangdong Province of China (No. 2019A1515011916 to YPL, No. 2021A1515011455 to BC); the Science and Technology Program of Guangzhou (No. 202102010012 to BC); the Special Research for Science and Technology of the Guangdong Provincial Hospital of Chinese Medicine (No. YN2019MJ08 to BC); and the Innovation Team Project of Educational Commission of Guangdong Province of China (No. 2021KCXTD020 to BC).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2282/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2282/coif). YPL reports the funding from National Natural Science Foundation of China (No. 82004385 to YPL) and the Natural Science Foundation of Guangdong Province of China (No. 2019A1515011916 to YPL). BC reports the funding from National Natural Science Foundation of China (No. 82174396 to BC), the Natural Science Foundation of Guangdong Province of China (No. 2021A1515011455 to BC), the Science and Technology Program of Guangzhou (No. 202102010012 to BC), and the Special Research for Science and Technology of the Guangdong Provincial Hospital of Chinese Medicine (No. YN2019MJ08 to BC). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hinai GA, Shandoodi MA, Sirasanagandla SR, et al. Radiologic evaluation of congenital anomalies of anterior and posterior arch of atlas in Omani subjects. Anat Cell Biol 2021;54:436-40. [Crossref] [PubMed]
- Junior MGP, Dos Santos NADSQ, Ribeiro RT, et al. Hypoplasia of C1's posterior arch: Is there an ideal anatomical classification? Surg Neurol Int 2021;12:623. [Crossref] [PubMed]
- Sawada H, Akiguchi I, Fukuyama H, et al. Marked canal stenosis at the level of the atlas. Neuroradiology 1989;31:346-8. [Crossref] [PubMed]
- Currarino G, Rollins N, Diehl JT. Congenital defects of the posterior arch of the atlas: a report of seven cases including an affected mother and son. AJNR Am J Neuroradiol 1994;15:249-54. [PubMed]
- Shah S, Dalvie S, Rai RR. Congenital malformed posterior arch of atlas with fusion defect: a case of developmental canal stenosis causing cervical myelopathy. J Spine Surg 2017;3:489-97. [Crossref] [PubMed]
- Bhattacharjee S, Mudumba V, Aniruddh PK. Spinal canal stenosis at the level of Atlas. J Craniovertebr Junction Spine 2011;2:38-40. [Crossref] [PubMed]
- Wang J, Zhu C, Li H, et al. Classification and Surgical Treatment of Developmental Spinal Canal Stenosis at Atlas Level: A 15-Case Study. Spine (Phila Pa 1976) 2021;46:1542-50. [Crossref] [PubMed]
- Musha Y, Mizutani K. Cervical myelopathy accompanied with hypoplasia of the posterior arch of the atlas: case report. J Spinal Disord Tech 2009;22:228-32. [Crossref] [PubMed]
- Phan N, Marras C, Midha R, et al. Cervical myelopathy caused by hypoplasia of the atlas: two case reports and review of the literature. Neurosurgery 1998;43:629-33. [Crossref] [PubMed]
- Hsu YH, Huang WC, Liou KD, et al. Cervical spinal stenosis and myelopathy due to atlas hypoplasia. J Chin Med Assoc 2007;70:339-44. [Crossref] [PubMed]
- Tokiyoshi K, Nakagawa H, Kadota T. Spinal canal stenosis at the level of the atlas: case report. Surg Neurol 1994;41:238-40. [Crossref] [PubMed]
- Banczerowski P, Czigléczki G, Papp Z, et al. Minimally invasive spine surgery: systematic review. Neurosurg Rev 2015;38:11-26; discussion 26. [Crossref] [PubMed]
- Reeves RA, DeWolf MC, Shaughnessy PJ, et al. Use of minimally invasive spine surgical instruments for the treatment of bone tumors. Expert Rev Med Devices 2017;14:881-90. [Crossref] [PubMed]
- Mayer HM. A History of Endoscopic Lumbar Spine Surgery: What Have We Learnt? Biomed Res Int 2019;2019:4583943. [Crossref] [PubMed]
- Jang JW, Lee DG, Park CK. Rationale and Advantages of Endoscopic Spine Surgery. Int J Spine Surg 2021;15:S11-20. [Crossref] [PubMed]
- McKay SD, Al-Omari A, Tomlinson LA, et al. Review of cervical spine anomalies in genetic syndromes. Spine (Phila Pa 1976) 2012;37:E269-77. [Crossref] [PubMed]
- Pascual-Gallego M, Budke M, Villarejo F. Spinal stenosis at the level of atlas in a boy with Down syndrome. A case report and literature review. Neurocirugia (Astur) 2014;25:29-32. [Crossref] [PubMed]
- Kawabori M, Hida K, Akino M, et al. Cervical myelopathy by C1 posterior tubercle impingement in a patient with DISH. Spine (Phila Pa 1976) 2009;34:E709-11. [Crossref] [PubMed]
- Pluemvitayaporn T, Kunakornsawat S, Piyaskulkaew C, et al. Chronic posterior atlantoaxial subluxation associated with os odontoideum: a rare condition. A case report and literature review. Spinal Cord Ser Cases 2018;4:110. [Crossref] [PubMed]
- Iki Y, Morofuji Y, Ozono K, et al. Concurrent Ossification of the Posterior Atlantoaxial Interlaminar Membrane and Atlas Hypoplasia: A Case Report. NMC Case Rep J 2020;7:147-50. [Crossref] [PubMed]
- Desai SK, Vadivelu S, Patel AJ, et al. Isolated cervical spinal canal stenosis at C-1 in the pediatric population and in Williams syndrome. J Neurosurg Spine 2013;18:558-63. [Crossref] [PubMed]
- Miyoshi Y, Yasuhara T, Date I. Noonan syndrome with occipito-atlantal dislocation and upper cervical cord compression due to C1 dysplasia and basilar invagination. Neurol Med Chir (Tokyo) 2011;51:463-6. [Crossref] [PubMed]
- Umebayashi D, Hara M, Nakajima Y, et al. Posterior fixation for atlantoaxial subluxation in a case with complex anomaly of persistent first intersegmental artery and assimilation in the C1 vertebra. Neurol Med Chir (Tokyo) 2013;53:882-6. [Crossref] [PubMed]
- Kim JB, Park SW, Lee YS, et al. Two Cases of Klippel-Feil Syndrome with Cervical Myelopathy Successfully Treated by Simple Decompression without Fixation. Korean J Spine 2015;12:225-9. [Crossref] [PubMed]
- Proietti L, Scaramuzzo L, Sessa S, et al. Cervical myelopathy due to ossification of the transverse atlantal ligament: a Caucasian case report operated on and literature analysis. Orthop Traumatol Surg Res 2012;98:470-4. [Crossref] [PubMed]
- Devi BI, Shenoy SN, Panigrahi MK, et al. Anomaly of arch of atlas--a rare cause of symptomatic canal stenosis in children. Pediatr Neurosurg 1997;26:214-7; discussion 217-8. [Crossref] [PubMed]
- Chung SB, Yoon SH, Jin YJ, et al. Anteroposterior spondyloschisis of atlas with incurving of the posterior arch causing compressive myelopathy. Spine (Phila Pa 1976) 2010;35:E67-70. [Crossref] [PubMed]
- Connor SE, Chandler C, Robinson S, et al. Congenital midline cleft of the posterior arch of atlas: a rare cause of symptomatic cervical canal stenosis. Eur Radiol 2001;11:1766-9. [Crossref] [PubMed]
- Liliang PC, Lui CC, Cheng MH, et al. Atlantal stenosis: a rare cause of quadriparesis in a child. Case report. J Neurosurg 2000;92:211-3. [PubMed]
- Kasliwal MK, Traynelis VC. Hypertrophic posterior arch of atlas causing cervical myelopathy. Asian Spine J 2012;6:284-6. [Crossref] [PubMed]
- Tang JG, Hou SX, Shang WL, et al. Cervical myelopathy caused by anomalies at the level of atlas. Spine (Phila Pa 1976) 2010;35:E77-9. [Crossref] [PubMed]
- Martin MD, Bruner HJ, Maiman DJ. Anatomic and biomechanical considerations of the craniovertebral junction. Neurosurgery 2010;66:2-6. [Crossref] [PubMed]
- Perera S, Davis CH, Gupta RC. Spinal cord compression caused by ossification of the transverse ligament of the atlas. Br J Neurosurg 1995;9:787-8. [Crossref] [PubMed]
- Geipel P. Studies on the fissure formation of the atlas and epistropheus. IV. Zentralbl Allg Pathol 1955;94:19-84. [PubMed]
- Notani N, Miyazaki M, Yoshiiwa T, et al. Dynamic paraspinal muscle impingement causing acute hemiplegia after C1 posterior arch laminectomy: A case report. Medicine (Baltimore) 2017;96:e9264. [Crossref] [PubMed]
- Ballhause TM, Velickovic M, Thiesen DM, et al. Congenital deformation of the posterior arch of the atlas: Subluxation of the atlanto-axial joint with temporary quadriplegia. SAGE Open Med Case Rep 2019;7:2050313X18823387.
- Yunoki M. A surgical case of C1 arch stenosis: A case report and review of literature. Surg Neurol Int 2021;12:71. [Crossref] [PubMed]
- Yamahata H, Hirano H, Yamaguchi S, et al. What Is the Most Representative Parameter for Describing the Size of the Atlas? CT Morphometric Analysis of the Atlas with Special Reference to Atlas Hypoplasia. Neurol Med Chir (Tokyo) 2017;57:461-6. [Crossref] [PubMed]
- Kelly MP, Oshima Y, Yeom JS, et al. Defining hyoplasia of the atlas: a cadaveric study. Spine (Phila Pa 1976) 2014;39:E1243-7. [Crossref] [PubMed]
- Hinck VC, Sachdev NS. Developmental stenosis of the cervical spinal canal. Brain 1966;89:27-36. [Crossref] [PubMed]
- Greenberg AD. Atlanto-axial dislocations. Brain 1968;91:655-84. [Crossref] [PubMed]
- McRAE DL. Bony abnormalities in the region of the foramen magnum: correlation of the anatomic and neurologic findings. Acta radiol 1953;40:335-54. [Crossref] [PubMed]
- Kim SW, Lee JH, Lee HW, et al. New Technique for C1 Double-Door Laminoplasty Using Allograft Spacers and Titanium Miniplate Screw Fixation: Technical Report. J Neurol Surg A Cent Eur Neurosurg 2016;77:155-60. [PubMed]
- Hott JS, Lynch JJ, Chamberlain RH, et al. Biomechanical comparison of C1-2 posterior fixation techniques. J Neurosurg Spine 2005;2:175-81. [Crossref] [PubMed]
- Yamahata H, Hiwatari T, Yonenaga M, et al. CT morphometric analysis of the cervical spinal canal with special reference to the correlation with the vertebral level. J Orthop Sci 2021;26:354-7. [Crossref] [PubMed]
- Yeom JS, Kafle D, Nguyen NQ, et al. Routine insertion of the lateral mass screw via the posterior arch for C1 fixation: feasibility and related complications. Spine J 2012;12:476-83. [Crossref] [PubMed]
- Yolcu YU, Helal A, Alexander AY, et al. Minimally Invasive Versus Open Surgery for Degenerative Spine Disorders for Elderly Patients: Experiences from a Single Institution. World Neurosurg 2021;146:e1262-9. [Crossref] [PubMed]
- Narain AS, Hijji FY, Duhancioglu G, et al. Patient Perceptions of Minimally Invasive Versus Open Spine Surgery. Clin Spine Surg 2018;31:E184-92. [Crossref] [PubMed]
- Ahn Y. The Current State of Cervical Endoscopic Spine Surgery: an Updated Literature Review and Technical Considerations. Expert Rev Med Devices 2020;17:1285-92. [Crossref] [PubMed]
- Sivakanthan S, Hasan S, Hofstetter C. Full-Endoscopic Lumbar Discectomy. Neurosurg Clin N Am 2020;31:1-7. [Crossref] [PubMed]
- Cacciola F, Phalke U, Goel A. Vertebral artery in relationship to C1-C2 vertebrae: an anatomical study. Neurol India 2004;52:178-84. [PubMed]
- Garrido BJ, Sasso RC. Occipitocervical fusion. Orthop Clin North Am 2012;43:1-9. vii. [Crossref] [PubMed]
- Ghasem A, Sharma A, Greif DN, et al. The Arrival of Robotics in Spine Surgery: A Review of the Literature. Spine (Phila Pa 1976) 2018;43:1670-7. [Crossref] [PubMed]
- Huang J, Li Y, Huang L. Spine surgical robotics: review of the current application and disadvantages for future perspectives. J Robot Surg 2020;14:11-6. [Crossref] [PubMed]
- Chen H, Zhu X, Dong L, et al. Study on robot-assisted pedicle screw implantation in adolescent idiopathic scoliosis surgery. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2021;35:1457-62. [PubMed]
- Lee NJ, Zuckerman SL, Buchanan IA, et al. Is there a difference between navigated and non-navigated robot cohorts in robot-assisted spine surgery? A multicenter, propensity-matched analysis of 2,800 screws and 372 patients. Spine J 2021;21:1504-12. [Crossref] [PubMed]
- Howard JJ, Abinahed J, Navkar N, et al. Robotic-assisted minimally invasive surgery of the spine (RAMISS): a proof-of-concept study using carbon dioxide insufflation for multilevel posterior vertebral exposure via a sub-paraspinal muscle working space. Int J Med Robot 2017; [Crossref] [PubMed]
- Dalton T, Sykes D, Wang TY, et al. Robotic-Assisted Trajectory Into Kambin's Triangle During Percutaneous Transforaminal Lumbar Interbody Fusion-Initial Case Series Investigating Safety and Efficacy. Oper Neurosurg (Hagerstown) 2021;21:400-8. [Crossref] [PubMed]
- Tsuruta W, Yanaka K, Okazaki M, et al. Cervical myelopathy caused by hypoplasia of the atlas and ossification of the transverse ligament--case report. Neurol Med Chir (Tokyo) 2003;43:55-9. [Crossref] [PubMed]
- Bokhari R, Baeesa S. Atlas hypoplasia and ossification of the transverse atlantal ligament: a rare cause of cervical myelopathy. Case Rep Neurol Med 2012;2012:893284. [Crossref] [PubMed]
(English Language Editor: B. Draper)



