
Clinical characteristics and surgical treatment of congenital cystic adenomatoid malformation in adults: the largest cohort of 46 patients
Introduction
Congenital cystic adenomatoid malformation (CCAM) is a rare congenital anomaly of the terminal respiratory structures (1,2), constituting up to 95% of congenital cystic lung lesions. Approximately 90% of CCAM lesions are detected during infancy. The diagnosis of CCAM in adults is extremely rare (3-5), with only 70 cases reported in English literature to date (6). The following sub-types of CCAM have been described (3): type I (75%) is characterized by the presence of one or several cysts between 2 to 10 cm; type II (10–15%) consists of numerous smaller cysts (~0.5–2 cm); type III (10%) features large cysts that are often mixed with non-cystic components. Some adult cases are asymptomatic and diagnosed by routine chest X-rays. Others may present with dyspnea, recurrent pulmonary infection, hemoptysis, hemithorax, or pneumothorax (6,7). Malignant transformation has also been reported, such as pleuropulmonary blastoma and bronchoalveolar carcinoma (2). On computed tomography (CT), CCAM lesions usually present as pulmonary cysts, but they may sometimes resemble bronchiectasis or lung tumors. The lack of specific imaging features and clinical manifestations makes diagnosis and treatment challenging for clinicians. Surgical resection, including lobectomy or wedge resection, is recommended for all patients to prevent recurrent pulmonary infections and possible malignant transformation (5,8-12).
Although CCAM in adults are always presented as case reports in the previous literature (13,14), there are few data on summary of clinical characteristics and surgical treatment in these rare lesions. Here, we report the largest case series of adult CCAM, with descriptions of patient demographics, medical history, preoperative investigations, intraoperative findings, and postoperative outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1747/rc).
Methods
Patients
We collected the files of 46 CCAM patients admitted to the Department of Thoracic Surgery at West China Hospital (a 4,300-bed major medical center in Chengdu, China) between February 2009 and March 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This case series study received ethical approval from the Institutional Review Board of West China Hospital of Sichuan University, China (No. 2019-1087). Individual consent for this retrospective analysis was waived.
Assessments
We assessed patients with demographics, medical history, preoperative investigations, intraoperative findings, and postoperative outcomes. All patients received routine laboratory testing, electrocardiography (ECG), pulmonary function tests (PFTs), and chest computed tomography (CT) before surgery. Patients with a history of tuberculosis were scrutinized for possible active tuberculosis. Fiberoptic bronchoscopy was used to evaluate the lesions if they were near the hilum. Sputum culture was completed for patients with a productive cough. Anti-tuberculous or other antibacterial treatment(s) were administered preoperatively if pathogenic bacteria were identified.
Surgery was performed if the following indications were present: (I) recurrent clinical symptoms; (II) the lesion was limited to one lobe; (III) co-existing cancer was suspected; (IV) there were no contraindications for surgery. Either wedge resection or lobectomy was performed based on the size and location of the lesion, as determined by chest CT. Wedge resection was performed for peripheral lesions <3 cm, while lobectomy was used for larger or central lesions.
A chest tube was inserted for every patient after surgery and was removed when daily drainage was <200 mL without identifiable air leakage. A chest X-ray was taken to confirm adequate lung expansion. All patients received intra- and postoperative prophylaxis with ceftriaxone. For patients with an anaphylactic response to β-lactams, clindamycin was administered instead. Patients were discharged from the hospital when fully recovered.
Statistical analysis
Statistical Product and Service Solutions (SPSS) software version 22.0 (SPSS, Inc., Chicago, IL, USA) was used to analyze the data. Descriptive statistics were used to analyze the categorical and continuous variables.
Results
Patient demographics are summarized in Table 1. A total of 46 patients (22 males and 24 females) were included. The median age at diagnosis was 42.9 years (15–70 years). CCAM lesions did not show any anatomical preferences in our cohort. While 17 out of 46 patients (37.0%) were asymptomatic, 29 patients (63.0%) presented with systemic and respiratory symptoms, including fever, productive cough, hemoptysis, and chest pain. On chest CT, CCAM lesions manifested with diverse features, including solid mass, cyst, cavitary lesion, bronchiectasis, pulmonary sequestration with aberrant artery, or pneumonic lesions (Figure 1). Some lesions had a mixture of different imaging features. All CCAM lesions were fully resected (35 cases underwent lobectomy, and 11 underwent wedge resection). Thirty-three patients (71.7%) presented with various degrees of pleural adhesions, which were separated during the surgery. Twelve patients with severe pleural adhesions underwent prolonged surgery. Twenty-nine patients (63.0%) had video-assisted thoracic surgery (VATS) while 17 patients (37.0%) received posterolateral thoracotomy (PLT). One patient who commenced VATS was switched to thoracotomy due to severe pleural adhesions. Histological examinations of the resected specimens revealed 26 cases of pure CCAM lesions and 20 cases of mixed CCAM with other diseases (six cases of pulmonary sequestration, six cases of pulmonary mycosis, five cases of pre-malignant or malignant lung lesions, one case of ectopic esophageal cyst, one case of tuberculosis, and one case of pulmonary lymphangioleiomyomatosis). Postoperative complications occurred in 4 patients (8.7%): 2 patients developed pneumothorax, 1 patient acquired pneumonia, and the fourth patient developed deep venous thrombosis (DVT). No intraoperative or postoperative deaths occurred.
Table 1
| Characteristics | Value (N=46) |
|---|---|
| Age (years), median [range] | 42.9 [15–70] |
| Gender, n (%) | |
| Male | 22 (47.8) |
| Female | 24 (52.2) |
| Smoking, n (%) | |
| Yes | 12 (26.1) |
| No | 34 (73.9) |
| Location of lesions, n (%) | |
| RUL | 8 (17.4) |
| RLL | 12 (26.1) |
| LUL | 11 (23.9) |
| LLL | 15 (32.6) |
| Clinical symptoms, n (%) | |
| None | 17 (37.0) |
| Cough + sputum production | 17 (37.0) |
| Cough + hemoptysis | 6 (13.0) |
| Chest pain | 6 (13.0) |
| Preoperative diagnosis, n (%) | |
| Bronchiectasis | 7 (15.2) |
| Pulmonary cyst | 6 (13.0) |
| Pulmonary tuberculosis | 2 (4.3) |
| Pulmonary sequestration | 3 (6.5) |
| Lung tumor | 28 (60.9) |
| Surgical therapy, n (%) | |
| Lobectomy | 35 (76.1) |
| Wedge resection | 11 (23.9) |
| Surgical procedure, n (%) | |
| VATS | 29 (63.0) |
| PLT | 17 (37.0) |
| Pleural adhesions, n (%) | |
| No | 14 (30.4) |
| Yes (LPA: SPA) | 32 (69.6) |
| Postoperative type, n (%) | |
| I | 11 (23.9) |
| II | 7 (15.2) |
| III | 28 (60.9) |
| Postoperative pathology, n (%) | |
| CCAM | 26 (56.5) |
| CCAM + PS | 6 (13.0) |
| CCAM + PM | 6 (13.0) |
| CCAM + EEC | 1 (2.2) |
| CCAM + PT | 1 (2.2) |
| CCAM + PLAM | 1 (2.2) |
| CCAM + LC | 5 (10.9) |
| Complications, n (%) | |
| Pneumothorax | 2 (4.3) |
| Pneumonia | 1 (2.2) |
| Deep venous thrombosis | 1 (2.2) |
| Mortality | 0 |
CCAM, congenital cystic adenomatoid malformation; RUL, right upper lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; VATS, video-assisted thoracic surgery; PLT, posterolateral thoracotomy; LPA, local pleural adhesion; SPA, severe pleural adhesion; PS, pulmonary sequestration; PM, pulmonary mycosis; EEC, ectopic esophageal cyst; PT, pulmonary tuberculosis; LC, lung cancer; PLAM, pulmonary lymphangioleiomyomatosis.
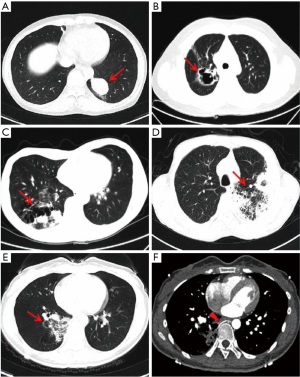
Among the five cases with coexisting pre-cancerous lesions or neoplasms, one patient was found to have atypical adenomatous hyperplasia (AAH), two had highly differentiated mucinous adenocarcinoma, one had highly differentiated adenocarcinoma, and one had pulmonary carcinoid tumor. The immunohistochemistry (IHC) of the pulmonary carcinoid tumor revealed moderate levels of phosphoenolpyruvate carboxykinase 1 (PCK1) but high expressions of neuroendocrine markers, such as chromogranin A (CgA), CD56, and synaptophysin. After discharge, all five patients were followed up regularly by medical oncologists at outpatient clinics, where they received periodic CT.
Discussion
CCAM is a rare congenital pulmonary disease. Most patients are diagnosed during infancy, and only a small proportion are diagnosed during adulthood. Adult patients with CCAM are often diagnosed during regular physical examination or due to recurrent pulmonary infection. In our study, 63.0% of the adult patients were diagnosed due to respiratory symptoms, such as cough, sputum, and hemoptysis. In contrast, almost all pediatric patients present with cough and dyspnea (15). In our study, the CT scans often showed the adult CCAM lesions as a solid mass or cyst, similar to imaging manifestations seen in pediatric cohorts (4,16). Other coexisting congenital anomalies, including pulmonary sequestration, heterotopic esophageal cyst, and arteriovenous malformation (AVM), have been reported (7,17-19). However, no patients had coexisting arteriovenous malformations in our cohort, which may reflect the possible difference between pediatric and adult cohorts.
Chest CT provides detailed information about the size and location of the lesions, which helps determine the surgical choice between wedge resection or lobectomy (20). Moreover, contrast CT may reveal aberrant vessels in patients with pulmonary sequestration. In our study, contrast CT identified that all six CCAM patients with pulmonary sequestration had abnormal arteries. These torturous abnormal arteries should be carefully handled during surgery to avoid excessive bleeding. Fiberoptic bronchoscopy was used to biopsy the lesions close to the bronchus and to determine if the bronchus was invaded by pathogens.
CCAM may increase the risk of infection by pathogenic microorganisms, including tuberculosis and other bacterial, fungal, hydatid, and viral infections. Although the risk is lower than pediatric cohorts, we suggest routine screening to prevent any severe infections superimposed by hospitalization and the stress of surgery (15).
Previous studies have reported several types of coexisting malignancies or transformations, such as pleuropulmonary blastoma, bronchoalveolar carcinoma, malignant mesenchymoma, and rhabdomyosarcoma (2,21,22). In our cohort, >10% of patients had coexisting pre-malignancies or malignancies. Additionally, our study is the first to report the coexistence of small pulmonary carcinoid tumor, a rare pathology, with adult CCAM. Thus, all CCAM specimens should be scrutinized extensively for underlying coexisting cancer. Currently, there are no guidelines on the follow-up for patients with comorbid CCAM and cancer. Thus, we recommend all adult CCAM patients be followed up in outpatient oncology clinics periodically to ensure no recurrence of CCAM or any associated neoplastic lesions.
A previous study of pediatric populations has suggested that type I CCAM is the most frequent (75%), whereas type III is the rarest (3). However, type III accounted for the majority of patients in our study (28 patients, 60.9%), which reflects a significant difference in pediatric and adult cohorts. While most type I and II lesions resemble non-specific inflammation, many type III CCAM lesions resemble tumors on CT. Future studies are needed to determine if type III CCAM lesions are more commonly associated with lung malignancies in adults.
Due to recurrent pulmonary infections and the increased risk of neoplastic transformation, surgical resection is the definitive treatment for CCAM (6,9,23). Both PLT and VATS are the mainstream surgical techniques. The choice between PLT and VATS is usually made based on the surgeon’s experience and the complexity of the specific case. During surgery, severe pleural and hilar adhesions may be found in some patients, possibly due to repeated infections. Adhesions may lead to incomplete resection of CCAM lesions, significant blood loss, and prolonged surgery. Extended procedures may thus increase the risk of postoperative complications, such as infections, sepsis, and respiratory distress.
Although CCAM appears to be a benign lesion with an excellent prognosis in our adult cohort, our small sample size made it difficult to determine if coexisting CCAM and lung malignancy represent a worse prognosis. Careful screening for underlying infections is also critical to ensure optimal patient outcomes, as shown in our cohort where no patient suffered from severe infectious complications or death. Future studies are needed to refine the guidelines for adult CCAM.
Conclusions
In summary, surgical resection is recommended for CCAM in adults and demonstrates an overall excellent prognosis. However, clinicians should be aware of possible coexisting infections and malignancies.
Acknowledgments
Funding: This study was supported by the Basic Science Foundation of Sichuan Province (No. 2019YJ0077 to F Lin), and the 1.3.5 Project for Disciplines of Excellence of Sichuan University (No. ZYJC18009 to J Mei).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1747/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1747/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1747/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of West China Hospital of Sichuan University, China (No. 2019-1087) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kravitz RM. Congenital malformations of the lung. Pediatr Clin North Am 1994;41:453-72. [Crossref] [PubMed]
- MacSweeney F, Papagiannopoulos K, Goldstraw P, et al. An assessment of the expanded classification of congenital cystic adenomatoid malformations and their relationship to malignant transformation. Am J Surg Pathol 2003;27:1139-46. [Crossref] [PubMed]
- Stocker JT, Madewell JE, Drake RM. Congenital cystic adenomatoid malformation of the lung. Classification and morphologic spectrum. Hum Pathol 1977;8:155-71. [Crossref] [PubMed]
- Rosado-de-Christenson ML, Stocker JT. Congenital cystic adenomatoid malformation. Radiographics 1991;11:865-86. [Crossref] [PubMed]
- Kwon YS, Koh WJ, Han J, et al. Clinical characteristics and feasibility of thoracoscopic approach for congenital cystic adenomatoid malformation in adults. Eur J Cardiothorac Surg 2007;31:797-801. [Crossref] [PubMed]
- Hamanaka R, Yagasaki H, Kohno M, et al. Congenital cystic adenomatoid malformation in adults: Report of a case presenting with a recurrent pneumothorax and a literature review of 60 cases. Respir Med Case Rep 2018;26:328-332. [Crossref] [PubMed]
- Oh BJ, Lee JS, Kim JS, et al. Congenital cystic adenomatoid malformation of the lung in adults: clinical and CT evaluation of seven patients. Respirology 2006;11:496-501. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 960-1. [Crossref] [PubMed]
- Liu C, Pu Q, Ma L, et al. Video-assisted thoracic surgery for pulmonary sequestration compared with posterolateral thoracotomy. J Thorac Cardiovasc Surg 2013;146:557-61. [Crossref] [PubMed]
- Singh R, Gogna P, Parshad S, et al. Video-assisted thoracic surgery for tubercular spondylitis. Minim Invasive Surg 2014;2014:963497. [Crossref] [PubMed]
- Scamporlino A, Ambrosini A, Turrini E, et al. Congenital cystic adenomatoid malformation in adults, presenting as a single cyst. Asian Cardiovasc Thorac Ann 2018;26:407-9. [Crossref] [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Aljarad B, Alkhayer I, Alturk A, et al. A rare case of congenital pulmonary airway malformation in a 14-year-old male presenting with spontaneous pneumothorax. Ann Med Surg (Lond) 2021;68:102692. [Crossref] [PubMed]
- Xu W, Wen Q, Zha L, Liu C, Huang P. Application of ultrasound in a congenital cystic adenomatoid malformation in an adult: A case report. Medicine (Baltimore) 2020;99:e23505. [Crossref] [PubMed]
- Parikh D, Samuel M. Congenital cystic lung lesions: is surgical resection essential? Pediatr Pulmonol 2005;40:533-7. [Crossref] [PubMed]
- Kim WS, Lee KS, Kim IO, et al. Congenital cystic adenomatoid malformation of the lung: CT-pathologic correlation. AJR Am J Roentgenol 1997;168:47-53. [Crossref] [PubMed]
- Samuel M, Burge DM. Management of antenatally diagnosed pulmonary sequestration associated with congenital cystic adenomatoid malformation. Thorax 1999;54:701-6. [Crossref] [PubMed]
- Khan NU, Jones MT, Greaves M. Case report: Congenital cystic adenomatoid malformation of an entire lung in a 33-year-old man: a case report and review of the literature. Br J Radiol 2008;81:e276-8. [Crossref] [PubMed]
- Zhang H, He X, Zhang S, et al. An adult patient with congenital pulmonary airway malformation and an esophageal cyst. Ann Transl Med 2019;7:396. [Crossref] [PubMed]
- Sarin YK, Sinha S, Bhalotra AR, et al. Giant Hydatid Cyst within a Congenital Cystic Adenomatoid Malformation of the Lung. APSP J Case Rep 2013;4:14. [PubMed]
- Ribet ME, Copin MC, Soots JG, et al. Bronchioloalveolar carcinoma and congenital cystic adenomatoid malformation. Ann Thorac Surg 1995;60:1126-8. [Crossref] [PubMed]
- Lantuejoul S, Ferretti GR, Goldstraw P, et al. Metastases from bronchioloalveolar carcinomas associated with long-standing type 1 congenital cystic adenomatoid malformations. A report of two cases. Histopathology 2006;48:204-6. [Crossref] [PubMed]
- Nishibayashi SW, Andrassy RJ, Woolley MM. Congenital cystic adenomatoid malformation: 1 30-year experience. J Pediatr Surg 1981;16:704-6. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


