
Preparation and identification of an anti-nicarbazin monoclonal antibody and its application in the agriculture and food industries
Introduction
Coccidiosis is an avian infectious disease caused by infection with Eimeria protozoa. Coccidiosis can cause extensive damage to the gastrointestinal tract of poultry, resulting in reductions in the nutrient absorption rate, feed conversion rate, body weight, egg production rate, and even death (1-3). Currently, 7 Eimeria parasites cause similar clinical symptoms of poultry coccidiosis infection (4). These protozoa can parasitize epidermal cells in the avian gut to elicit pathological reactions, such as partial damage to the intestinal mucosal barrier, blood loss, and shock syndrome (5-7). Coccidiosis is estimated to cause over £2 billion of damage to the global broiler farming industry each year (8).
Since the discovery of the anticoccidial activity of sulfonamides, nearly 50 anticoccidial drugs have been developed and are now used to prevent and treat avian coccidiosis (9). These drugs have made a major positive contribution to the poultry industry. However, with the long-term use of some of these drugs, the parasite population has developed drug resistance, thus reducing the drug-use cycle and efficacy (10-12). Nicarbazin, a common anticoccidial drug, has been widely used as a feed additive in broiler breeding because of its high broad-spectrum efficiency, non-toxicity, minimal immunosuppressive effects, and low number of resistant strains (13-15). As a drug treatment, generally given alone, it has been reported that the therapeutic effect of nicarbazine combined with salinomycin is better (16). As an equimolar complex of 4,4'-dinitrocarbanilide (DNC) and 2-hydroxy-4,6-dimethylpyrimidine (HDP), nicarbazin is an oxidative phosphorylation uncoupler that interferes with mitochondrial metabolism (see Figure 1) (17). DNC is the main active ingredient in nicarbazin and is active against coccidian (18). DNC has low solubility in water (i.e., 0.02 mg/L). Conversely, HDP is a hydrophilic compound with no anticoccidial activity that helps the body absorb DNC. The anticoccidial effect of the DNC-HDP complex is 10 times that of DNC alone (19). HDP is mainly excreted in the urine and is quickly eliminated after drug withdrawal. However, DNC becomes more concentrated in the liver and kidneys over time. DNC is slowly excreted through the feces, and the residual amount of DNC in the body after drug withdrawal is much higher than that of HDP (20). Thus, the Food and Agriculture Organization (FAO)/World Health Organization (WHO) of the United Nations uses DNC as a residual marker of nicarbazin.
Nicarbazin is an efficient and reliable drug that effectively prevents the widespread infection of poultry by coccidiosis in high-density poultry farming. However, the use of such drugs can lead to residues of the drug in animal-derived foods, which may compromise human food safety (21). Studies on the physiological toxicity of nicarbazine show that long-term intake of 80 times the normal dose will significantly increase the rate of sperm aberration in male rats, and long-term consumption of chicken, eggs and other foods left by nicarbazine may have certain risks to health, and its potential human side effects can not be ignored. The Codex Aliment Commission (CAC) has defined maximum residue limits (MRLs) for veterinary drugs in animal-derived foods and established a classification of pharmaceutically active substances and their MRLs in animal-derived foods. Excessive use of nicarbazin may poison the body, affect body temperature regulation, and increase the probability of heat stress effects (22). Chronic toxicological tests in mice and dogs have shown that the most significant effects of long-term nicarbazin use are weight loss and changes in vital organs, such as the liver and kidneys. In 1999, based on a toxicity assessment, the CAC stated that the maximum residue concentration of nicarbazin in broiler chickens should not exceed 200 µg/kg, and the acceptable daily intake is 400 µg/kg (23). To date, no reports have shown that nicarbazin causes serious toxic effects in humans; however, it is unclear whether the long-term intake of nicarbazin in low doses causes chronic poisoning in humans. Thus, many countries closely monitor the residues of anticoccidial drugs in animal-derived foods. In 2002, FAO/WHO banned the use of nicarbazine in imported food of animal origin. Japan has successively announced that the MRL of nicarbazine in imported poultry meat is 0.2 mg/kg. The MRL of nicarbazine in chicken tissues is stipulated as 0.2 mg/kg in China’s National Food Safety Standard-maximum residue limits for veterinary drugs in food (GB 31650-2019). Unfortunately, the current lack of rapid and efficient detection methods makes it difficult to monitor the residues of anticoccidial drugs appropriately.
In recent years, methods such as high-performance liquid chromatography (HPLC), gas chromatography, and liquid chromatography-tandem mass spectrometry have been widely used to detect nicarbazin residues (24). As the operation of such methods is complicated, they are not suitable for use in large-scale, high-throughput detection processes, and pose challenges for rapid detection. Analytical methods based on enzyme-linked immunosorbent assays (ELISAs) have been growing in use as rapid quantitative detection procedures of veterinary drug residues in animal-derived foods because of their high throughput, high efficiency, and low cost (25-27). ELISAs avoid the need for complex pre-processing steps, substantially increase testing throughput, eliminate the need for expensive equipment, and produce results that are easy to interpret. The detection of nicarbazin residues via an ELISA method requires the production of a specific antibody against DNC (28). However, as a small molecular drug, nicarbazine does not have immunogenicity, so it is necessary to carry out molecular modification of nicarbazine to make nicarbazine immunogenic. Small molecular modification requires that the immune site of nicarbazine should be fully exposed. If the characteristics of nicarbazine itself can not be changed, the synthetic modification of nicarbazine small molecule hapten was carried out by computer simulation, and the immunogenic nicarbazine hapten was obtained. So, there are few reports on ELISAs of nicarbazine in chicken. Huet reported in 2005 (29), Wang reported in 2014 that an ELISA assay for nicarbazine was developed, the detection limit in chicken is 9.2 ng/mL, the recovery rate is between 49.4% and 118%, and the intra-and inter-batch coefficients of variation are less than 20% (30). And our prepared anti-nicarbazin monoclonal antibody had a high sensitivity and specificity and wide applicability, the half-maximal inhibitory concentration (IC50) is 0.825 ng/mL, and the curve range was 0.3–24.3 ng/mL. The linearity of the standard curve was 99.51%. The recovery rate is 74.4–111.7%. The present study lays the foundations for developing an ELISA as an anticoccidial drug residue detection method with high sensitivity and specificity and wide applicability. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1452/rc).
Methods
BALB/c mice (license number: SCXK2015-0034) were purchased from SPF Biotechnology Co., Ltd. (Beijing, China). DNC and HDP standards were provided by Dr. Ehrenstorfer GmbH (Augsburg, Germany). Complete/incomplete Freund’s adjuvant, ELISA-coating buffer (20×), phosphate-buffered saline (PBS) (10×), tris-buffered saline tween washing solution (10×), blocking solution [containing bovine serum albumin (BSA)], ELISA-3,3',5,5'-tetramethylbenzidine (EL-TMB) colorimetric kits, ELISA stop buffer, and goat anti-mouse immunoglobulin G (IgG) were obtained from Shanghai Shenggong (Shanghai, China). Hypoxanthine-thymidine and hypoxanthine-aminopterin-thymidine (HAT) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Synthesis of nicarbazin hapten derivatives
The primary condition for obtaining a monoclonal antibody against DNC is the preparation of an appropriate nicarbazin antigen. Because the metabolite DNC, which is formed after nicarbazin entry into the body, is the residual marker of nicarbazin, we prepared a DNC hapten product using DNC, pyridine, and 5-amino-2-nitro benzoic acid as the raw materials.
Methyl (4-nitrophenyl)carbamate (1.0 g) was dissolved in pyridine (50 mL), and 5-amino-2-nitrobenzoic acid (1.02 g) was then added. The reaction was stirred to dissolve and clarify the mixture. The reaction was then heated in an oil bath under reflux for 24 h. After stopping the reaction, the pyridine was removed by evaporation using a rotary evaporator, steaming, and drying to produce a red oily substance. The product was purified by 200–300 mesh silica gel column chromatography using petroleum ether/ethyl acetate (v/v, 1/1) as the eluent. After purification, the carboxynicarbazine hapten product was obtained. A nuclear magnetic resonance (NMR) analysis was conducted to confirm that the correct product was obtained.
Nicarbazin holoantigen synthesis
The DNC hapten cannot be used directly for immunization and must be coupled with a suitable carrier protein. In this study, DNC hapten was coupled with BSA to produce the immunogen DNC-BSA and also with ovalbumin (OVA) to produce the coating antigen DNC-OVA. The carboxynicarbazine hapten (13 mg) was dissolved in dimethyl sulfoxide (DMSO), and carbodiimide (8.6 mg) was then added. The solution was stirred at room temperature for 1 h. N-hydroxysuccinimide (NHS) (5.2 mg) was added, and the mixture was stirred for a further 2 h to obtain the reaction liquid A. BSA (50 mg) was dissolved in PBS (pH 7.4) to obtain liquid B. Liquid A was slowly added dropwise to liquid B at room temperature with stirring for 4 h, and then subjected to 0.02 mol/L PBS dialysis purification. Carboxynicarbazine hapten (5.7 mg) was dissolved in acetone and triethylamine (20 μL), and the mixture was stirred at room temperature for 30 min. Isobutyl chloroformate (2.7 μL) was added, and the solution was stirred for 2 h to obtain the hapten activator solution A. OVA (50 mg) was dissolved in carbonate buffer to obtain solution B, and solution A was added dropwise to solution B with stirring for 4 h. This sample was then subjected to 0.02 mol/L PBS dialysis purification. The ratios of hapten, carrier protein, and conjugated product used in synthesizing the nicarbazin-conjugated antigen were used to calculate the binding ratios by comparing the absorbance values of the hapten, carrier protein, and the conjugated product at 260 and 280 nm.
Hybridoma cell preparation
A total of 15 BALB/c female mice aged 6–8 weeks were immunized with 15 DNC-BSA antigens. All animals were kept in a pathogen-free environment and fed ad lib. All animal experiments followed the GB/T 35892-2018 guidelines for Ethical Review of Experimental Animal Welfare, and were completed under the guidance of the Experimental Animal Administration and use Committee (IACUC) of Zhejiang Institute for Food and Drug Control [No. SYXK(Zhe) 2021-0010]. The mice were divided into the E002, E013, and E020 groups (5 mice per group). DNC-BSA was fully emulsified with the same amount of Freund’s complete adjuvant, and the mice were subcutaneously injected with 0.2 mL of the emulsion. Immunization was enhanced every 2 weeks. Freund’s complete adjuvant was replaced by Freund’s incomplete adjuvant. The subsequent method and dose were the same as those for the first immunization. Fundus venous blood was collected 1 week after the last intensive immunization, and the inhibition titer was measured. The final immunization was carried out when the titer was above 1:10,000. Next, the immunogen solution (0.1 mL) without any adjuvant was injected intraperitoneally. The mice were sacrificed 3 days later, and their spleens were fused with myeloma cells. Mice that have not been immunized successfully should be treated innocuously, in accordance with the regulations of Beijing Municipal regulations on Animal epidemic Prevention, be innocuously executed and handed over to a professional harmless treatment company for treatment. A protocol was prepared before the study without registration.
Each mouse was subjected to tail-vein blood sampling. The serum was separated from the blood samples, and 4 µL of mouse immune serum was diluted at 1:100, 1:800, 1:1,600, 1:3,200, 1:6,400, 1:8,400, and 1:12,800; negative serum from an untreated control mouse was diluted at 1:1,600. An indirect ELISA method was performed to determine the serum titer, which was set as the highest dilution ratio of serum corresponding to an optical density at 450 nm (OD450) value ≥2.1 times the OD450 value for the negative control well [i.e., positive (P)/negative (N) ≥2.1]. Thus, a result was deemed positive when the P/N value was >2.1. A P/N value of >3 after diluting the mouse immune serum at 1:12,800 was sufficient to use as a sample in the cell fusion experiment. The E013-1 mouse with the highest serum titer was selected for the cell fusion experiment. Cell fusion was performed using myeloma cells from SP2/0 mice and splenocytes from the E013-1 mouse (31). Positive wells with the highest OD450 values were selected to generate 3rd subclones.
Suspensions of 2–3×107 myeloma cells and 1×108 spleen B lymphocytes were transferred to a 50 mL centrifugal tube, supplemented with 30 mL of incomplete culture medium, centrifuged, and the supernatant was discarded. The bottom of the tube was struck lightly to loosen the cell mass, and 1 mL of preheated polyethylene glycol (PEG) fusion agent was added slowly to the cells along the wall. The centrifugal tube was rotated along the side, and left to stand for 60 s, after which 20 mL of incomplete culture medium was added. The incomplete culture medium diluted the PEG and reduced its melting-promoting effect. The supernatant was discarded after centrifugation, and HAT-containing complete medium was added to make the cell suspension. The cell suspension was added to a 96-well cell culture plate and placed at 37 ℃ in a 5% carbon dioxide cell culture box.
The unfused myeloma cells and unfused lymphocytes gradually died when cultured in the HAT medium, while the fused hybridoma cells survived and proliferated in this medium. After 7–10 days of incubation, the DNC-OVA antigen was used to coat the enzyme label plate. For this process, the DNC-OVA antigen was diluted by 500, 1,000, and 2,000 times the concentration, and 100 μL was added to each well and incubated at 37 ℃ for 2 h. The plate was washed once and patted dry. The plate was then sealed with 2% BSA by adding 150 μL of the BSA sample per well, incubating in the incubator at 37 ℃ for 2 h, and finally pouring out the deionized water in the plate hole and patting it dry with absorbent paper. Cell supernatants from the 96-well plate were absorbed and added to the sealed enzyme label plate. The cells were incubated at 37 ℃ for 30 min. The plates were washed 5 times and patted dry. Sheep anti-rat IgG labeled with horseradish peroxidase (HRP) was incubated at 37 ℃ for 30 min, using the deionized water washed 5 times, and pat it dry with absorbent paper. The reaction was terminated by adding 100 μL of TMB color solution per well, followed by incubation at 37 ℃ for 15 min, and the addition of 50 μL of 2M sulfuric acid per well. The extent of the reaction was determined by an enzyme labeling instrument (wavelength 450/630 nm). The ELISA-positive clones were selected and added to a 24-well cell culture plate and cultured. The ELISA-positive clones were re-screened 3 days later. The Western blot (WB) positive clones were re-screened. The WB positive cells were subcloned. After 3 subclones, the monoclonal cells that stably secreted antibodies were screened out. The positive clones were expanded and cultured and then fixed and frozen. Ascites antibodies were prepared by in-vivo induction. Balb/c mice (8-week-old) were injected with 0.5 mL of sterilized paraffin oil intraperitoneally. Hybridoma cells (5×105 cells) were injected intraperitoneally 7 days later. Ascites were collected 7 days later. The nicarbazin monoclonal antibody solution was purified using the octanoic acid-saturated ammonium sulfate method.
Statistical analysis
The optimal ascites antibody was selected, and the indirect competitive ELISA method was used to optimize the kit reaction system, including the parameters of the coating antigen concentration, the dilution ratio of the antibody working fluid, and the curve range of the standard sample. Finally, the ELISA method for nicarbazin was established. The stability of the anti-nicarbazin monoclonal antibody in the ELISA assay was tested with initial experiments measuring the intra- and inter-plate variation coefficients (32). The cross-reaction rate is commonly used as the evaluation criterion. In this experiment, nicarbazin, diclazuril, salinomycin, dinitrotoxamine, monensin, ethoxyamide benzyl ester, and sulfaquinoxaline were diluted, and indirect competition with the monoclonal antibodies was analyzed by ELISA. A standard curve was generated, and IC50 values were obtained by analysis. The cross-reaction rate was then calculated using the following formula: (the nicarbazin concentration causing 50% inhibition/concentrations of other anticoccidial drugs causing 50% inhibition) × 100%.
For the immunoassay methods, additive recovery experiments are often performed to test the accuracy of the established method. Because nicarbazin is used mainly as a feed additive in poultry farming, different chicken, duck, and egg samples were used to determine the fortified recovery of the anti-nicarbazin monoclonal antibody. A high-standard solution (1 mg/mL) was diluted with methanol to 10 μg/mL. A sample (1 g) was weighed and added to 0 or 30 μL of the high-standard solution (10 μg/mL). Next, 5 mL of methanol was added, and the mixture was centrifuged at 3,000 rpm for 5 min with uniform oscillation. The supernatant was then separated and diluted 30 times for detection.
The OD value of the supernatant sample was measured using an indirect competitive ELISA to calculate the inhibition rate, which was taken into the standard curve regression equation to calculate the fortified recovery using the following formula: fortified recovery (%) = [fortified value (ng/g)/measured value (ng/g)] × 100%.
Results
NMR identification of nicarbazin hapten derivative
Figure 2 shows the NMR spectrum of the DNC hapten. 1H NMR (400 MHz, DMSO-d6): δ 13.80 (s, 1H, COOH), 9.74 (s, 1H, NH), 9.73 (s, 1H, NH), 8.23 (d, J=8.9 Hz, 2H, ArH), 8.06 (d, J=8.9 Hz, 1H, ArH), 7.90 (d, J=2.3 Hz, 1H, ArH), and 7.78–7.69 (m, 3H, ArH). The signal at δ=13.80 ppm represents the carboxy hydrogen on the DNC hapten, and the signal at δ=7.90 ppm represents the hydrogen on the amide. The presence of these characteristic peaks confirmed the structure of the DNC hapten product.

Identification of nicarbazin holoantigen
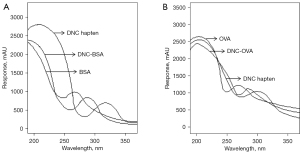
The matrix-assisted laser desorption/ionization time-of-flight mass spectrometry results for DNC-OVA and DNC-BSA are shown in Figure 3. In the ultraviolet spectrum, the OVA ultraviolet absorption peak was at 270 nm, and the DNC hapten absorption peak was at 310 nm. The absorption peak of the DNC-OVA coupling product fell between the 2, indicating that the DNC hapten had successfully coupled with the carrier protein OVA. Similarly, the absorption peak of the DNC-BSA coupling product was located between the absorption peaks for the DNC hapten and the carrier protein BSA, demonstrating the successful coupling of the DNC hapten and the carrier protein BSA (see Figure 4).

Trinitrobenzene sulfonic (TNBS) acid only reacts with the N-terminal α-amino and lysine ε-amino groups in proteins (optimally at pH 9–10), generating a yellow complex. The carrier proteins BSA and OVA contain an N-terminal α-amino group and lysine residues. We found 2 distinct absorption peaks at 350 and 430 nm, indicating that the lysine ε-amino groups in the proteins and the ε-amino group in free lysine react equally well with TNBS and have the same spectral characteristics. The α-amino and lysine ε-amino groups in proteins also react equivalently with TNBS in a slightly alkaline solution. Conversely, when the hapten structure does not contain an amino group or contains only 1 amino group, TNBS reacts with the amino group on the carrier protein, and the equivalent amino group is consumed. Thus, the hapten coupling ratio can be determined by comparing the numbers of amino groups on the carrier protein before and after the coupling reaction. The coupling ratios of the DNC hapten to the carrier proteins BSA and OVA were 10.45 and 7.28, respectively.
Screening for monoclonal antibodies
The hybridization fusion assay revealed that the resulting fusion rate was approximately 80%, and the positive rate was approximately 15%. To generate the 3rd subclones, the 7 positive wells with the highest OD450 values were selected, and 4 hybridoma cell strains were ultimately generated that stably secreted specific anti-DNC antibodies. These cell lines were named 2B5-F3-C12, 2B5-G1-G7, 2B5-C3-G1, and 2B5-F3-E8. The hybridoma cell strains were cultured continuously for 1 month, then frozen and re-cultured after resuscitation from cryopreservation for 1 month, after which all 4 hybridoma cell strains still secreted monoclonal antibodies with a stable antibody level. These 4 hybridoma cell strains were separately used to prepare mouse ascites, and a total of 9 ascites samples were obtained from the 4 cell strains. The antibody titers of the ascites were detected using the indirect ELISA method. The titers of the ascites induced by the 4 hybridoma cell strains were each higher than 104, and the MC-8 and MC-9 antibody titers produced by the 2B5-G1-G7 cell strain reached a maximum of 160,000, which is a sufficient level for an ELISA to detect DNC (see Figure 5).
ELISA kit parameters
The stability of the anti-nicarbazin monoclonal antibody MC-8 in an ELISA assay was tested. We coated 7 96-well plates with the anti-nicarbazin monoclonal antibody MC-8. Next, 1 column was drawn from each plate to detect the inter-plate variation coefficient, and 6 columns were drawn from the same plate to detect the intra-plate variation coefficient. The detection results showed that the intra- and inter-plate variation coefficients were <15%. For the ELISA method, the use of different matrices can affect the test results. Thus, a standard inhibition curve of different samples needs to be determined before actual sample detection, and the standard solution needs to be re-prepared to determine the standard curve for each test. The standard curve was tested in parallel for the monoclonal antibody, and the results showed that the linearity of the standard curve was 99.51%, the curve range was 0.3–24.3 ng/mL, and the IC50 value was 0.825 ng/mL.
The cross-reaction rates were 100% nicarbazin, <1% dichlorvos, <1% salinomycin, <1% dinitrotoxamine, <1% monensin, <1% ethoxyamide benzyl ester, and <1% sulfaquinoxaline. The antibody did not cross-react with other anticoccidial drugs, including dichlorvos, salinomycin, dinitrotoxamine, monensin, ethoxyamide benzyl ester, and sulfaquinoxaline. The antibody specifically binds to nicarbazin. The recovery results showed that the fortified recovery of the 15 chicken and duck samples ranged from 74.4–111.7%, while the fortified recovery of the 4 egg samples ranged from 81.2–86.9%.
Discussion
An ELISA-based method has potential application in the detection of nicarbazin residues. To develop such an assay, a suitable monoclonal antibody directed against DNC must be prepared. DNC is the primary residue of nicarbazin in poultry. According to the Landsteiner hapten theory, the molecular weight of DNC is too small to be immunogenic (33). Thus, DNC needs to be coupled with macromolecules to stimulate an immune response in animals. In the present study, DNC carboxyl hapten products were prepared from DNC, pyridine, and 5-amino-2-nitrobenzoic acid. After a small molecule hapten combines with a carrier protein, its immunogenicity is enhanced; however, as the application purpose of the prepared antibody is different, a corresponding immunization procedure is required. Because the antibodies prepared in this study were mainly used for detection, multiple immunizations were administered at multiple subcutaneous points, and the immunization cycle was lengthened to reduce adjuvant adverse reactions and immune tolerance.
We obtained immunized mice with relatively high antibody titers through basic and booster immunizations. The efficiency of cell fusion is key to preparing a high-titer monoclonal antibody. For example, selecting a chemical derivation method should consider both the compound concentration and its toxicity to cells. In this study, 50% PEG2000 was used, and the fusion rate was up to 80%. The traditional enzyme-labeled antibody method (the sodium-periodate method) requires the molar ratio of enzyme to antibody in the reaction system to be 4:1. As HRP binds to many sites on an antibody under strongly oxidizing conditions, the activated HRP can connect multiple molecules. This reduces the enzyme activity of the enzyme-labeled products and enables the prepared conjugates to mix with many polymers. Thus, we developed a method that improves on the traditional methods.
Under our method (I) the process of blocking the amino group was omitted, as there were few free amino groups present, and (II) the molar ratio of HRP to antibody was reduced to 2:1. Our improved method is simpler than traditional methods, and the loss of enzyme activity observed under conventional methods was reduced. The sensitivity of the developed nicarbazin ELISA kit was 0.3 ng/mL, which is 10 times better than that of the reported ELISAs (34). The specificity was verified, as there was no cross-reaction with 6 similar coccidial drugs (i.e., diclazuril, salinomycin, dinitrotropamine, monensin, ethoxyamide benzyl ester, and sulfaquinoxaline).
Conclusions
In this study, a synthetic route for a nicarbazin marker DNC antigen was established, and hybridoma cell lines and monoclonal antibodies with a high titer, good specificity, and no cross-reactivity with other anticoccidial drugs were prepared. The parameters of an ELISA kit were established, including the detection standard curve, sensitivity, recovery, and cross-reaction rate. The antigen synthesis route and the precise molecular structures of the intermediate metabolites of nicarbazin were explored.
The stability and accuracy of the kit detection method depend on the stability of the antibody, and there is instability between the antibodies prepared by different batches of mouse ascites, so the variable region of the antibody selected in this study will be sequenced in the h follow-up research plan. The recombinant antibody was prepared by cell expression method, and a cell expression system capable of expressing active protein sites was established. The stable expression of recombinant antibody with high sensitivity was obtained and the stable production of antibody was realized.
Currently, many countries have earmarked the monitoring of veterinary drug residues as a high priority and have clearly required the implementation of monitoring and testing standards for drug residues in animal products. In the past 2 years, the CAC has established residue limit standards for 3,724 agricultural and veterinary drugs across 185 types of drugs. The ELISA detection methods and kit products developed in this study provide a rapid, sensitive, accurate and effective technical support for the detection of nicarbazine residues in animal food.
Acknowledgments
Funding: This research was funded by the Key R&D Program of Zhejiang Province (No. 2021C02062 and No. 2021C03088) and the National Key R&D Program of China (No. 2018YFC01603400).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1452/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1452/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1452/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments followed the GB/T 35892-2018 guidelines for Ethical Review of Experimental Animal Welfare, and were completed under the guidance of the Experimental Animal Administration and use Committee (IACUC) of Zhejiang Institute for Food and Drug Control [No. SYXK(Zhe) 2021-0010].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blake DP, Tomley FM. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol 2014;30:12-9. [Crossref] [PubMed]
- Blake DP, Knox J, Dehaeck B, et al. Re-calculating the cost of coccidiosis in chickens. Vet Res 2020;51:115. [Crossref] [PubMed]
- Yu H, Zou W, Xin S, et al. Association Analysis of Single Nucleotide Polymorphisms in the 5' Regulatory Region of the IL-6 Gene with Eimeria tenella Resistance in Jinghai Yellow Chickens. Genes (Basel) 2019;10:890. [Crossref] [PubMed]
- Allen PC, Fetterer RH. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev 2002;15:58-65. [Crossref] [PubMed]
- Sokale AO, Williams CJ, Hoerr FJ, et al. Effects of administration of an in ovo coccidiosis vaccine at different embryonic ages on vaccine cycling and performance of broiler chickens. Poult Sci 2021;100:100914. [Crossref] [PubMed]
- Guo A, Cai J, Gong W, et al. Transcriptome analysis in chicken cecal epithelia upon infection by Eimeria tenella in vivo. PLoS One 2013;8:e64236. [Crossref] [PubMed]
- Wang X, Zou W, Yu H, et al. RNA Sequencing Analysis of Chicken Cecum Tissues Following Eimeria tenella Infection in Vivo. Genes (Basel) 2019;10:420. [Crossref] [PubMed]
- Williams RB. A compartmentalised model for the estimation of the cost of coccidiosis to the world's chicken production industry. Int J Parasitol 1999;29:1209-29. [Crossref] [PubMed]
- Chapman HD, Rathinam T. Focused review: The role of drug combinations for the control of coccidiosis in commercially reared chickens. Int J Parasitol Drugs Drug Resist 2022;18:32-42. [Crossref] [PubMed]
- Chapman HD. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol 1997;26:221-44. [Crossref] [PubMed]
- Joyner LP. Coccidiosis: problems arising from the development of anticoccidial drug resistance. Exp Parasitol 1970;28:122-8. [Crossref] [PubMed]
- Ryley JF. Drug resistance in coccidia. Adv Vet Sci Comp Med 1980;24:99-120. [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis V, Azimonti G, et al. Safety and efficacy of a feed additive consisting of lasalocid A sodium and nicarbazin (Nilablend™ 200G) for chickens for fattening (Zoetis Belgium SA). EFSA J 2021;19:e06466.
- EC. Commission Regulation (EU) No 875/2010 of 5 October 2010 concerning the authorisation for 10 years of an additive in feedingstuffs. Off J Eur Union. 2010;263:4-6.
- Bilandžić N, Dolenc J, Gačnik KŠ, et al. Feed additives diclazuril and nicarbazin in egg and liver samples from Croatian farms. Food Addit Contam Part B Surveill 2013;6:90-7. [Crossref] [PubMed]
- Vereecken M, Dehaeck B, Berge AC, et al. Synergistic effect of a combination of nicarbazin and monensin against coccidiosis in the chicken caused by Eimeria spp. Avian Pathol 2020;49:389-93. [Crossref] [PubMed]
- Matabudul DK, Crosby NT, Sumar S. A new and rapid method for the determination of nicarbazin residues in poultry feed, eggs and muscle tissue using supercritical fluid extraction and high performance liquid chromatography. Analyst 1999;124:499-502. [Crossref] [PubMed]
- Bacila DM, Feddern V, Mafra LI, et al. Current research, regulation, risk, analytical methods and monitoring results for nicarbazin in chicken meat: A perspective review. Food Res Int 2017;99:31-40. [Crossref] [PubMed]
- Beier RC, Ripley LH, Young CR, et al. Production, characterization, and cross-reactivity studies of monoclonal antibodies against the coccidiostat nicarbazin. J Agric Food Chem 2001;49:4542-52. [Crossref] [PubMed]
- Goetting V, Lee KA, Tell LA. Pharmacokinetics of veterinary drugs in laying hens and residues in eggs: a review of the literature. J Vet Pharmacol Ther 2011;34:521-56. [Crossref] [PubMed]
- Lima AL, Barreto F, Rau RB, et al. Determination of the residue levels of nicarbazin and combination nicarbazin-narasin in broiler chickens after oral administration. PLoS One 2017;12:e0181755. [Crossref] [PubMed]
- Bacila DM, Feddern V, Mafra LI, et al. Current research, regulation, risk, analytical methods and monitoring results for nicarbazin in chicken meat: A perspective review. Food Res Int 2017;99:31-40. [Crossref] [PubMed]
- Programming for adolescent health and development. Report of a WHO/UNFPA/UNICEF Study Group on Programming for Adolescent Health. World Health Organ Tech Rep Ser 1999;886:i-vi, 1-260. [PubMed]
- Wang B, Liu J, Zhao X, et al. Determination of Eight Coccidiostats in Eggs by Liquid-Liquid Extraction-Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2020;25:987. [Crossref] [PubMed]
- Stanker LH, Elissalde MH, Rowe LD, et al. Detection of coccidiostats by immunoassay. Food and Agricultural Immunology 1994;6:45-54. [Crossref]
- Beier RC, Stanker LH. An antigen based on molecular modeling resulted in the development of a monoclonal antibody-based immunoassay for the coccidiostat nicarbazin. Anal Chim Acta 2001;444:61-7. [Crossref]
- Hagren V, Crooks SR, Elliott CT, et al. An all-in-one dry chemistry immunoassay for the screening of coccidiostat nicarbazin in poultry eggs and liver. J Agric Food Chem 2004;52:2429-33. [Crossref] [PubMed]
- Connolly L, Fodey TL, Crooks SR, et al. The production and characterisation of dinitrocarbanilide antibodies raised using antigen mimics. J Immunol Methods 2002;264:45-51. [Crossref] [PubMed]
- Huet AC, Mortier L, Daeseleire E, et al. Screening for the coccidiostats halofuginone and nicarbazin in egg and chicken muscle: development of an ELISA. Food Addit Contam 2005;22:128-34. [Crossref] [PubMed]
- Wang HJ, Liu ZH, Wang X, et al. Enzyme-linked Immunosorbent Assay for Detection of Nicarbazin Residue in Chicken Muscle. Chinese Journal of Veterinary Drug 2014;48:59-61.
- Sakata R, Shoyama Y, Murakami H. Production of monoclonal antibodies and enzyme immunoassay for typical adenylate cyclase activator, Forskolin. Cytotechnology 1994;16:101-8. [Crossref] [PubMed]
- Liu C, Chhabra GS, Zhao J, et al. Comparison of Laboratory-Developed and Commercial Monoclonal Antibody-Based Sandwich Enzyme-Linked Immunosorbent Assays for Almond (Prunus dulcis) Detection and Quantification. J Food Sci 2017;82:2504-15. [Crossref] [PubMed]
- Coleman MR, Rodewald JM, Brunelle SL, et al. Determination and confirmation of nicarbazin, measured as 4,4-dinitrocarbanilide (DNC), in chicken tissues by liquid chromatography with tandem mass spectrometry: First Action 2013.07. J AOAC Int 2014;97:630-40. [Crossref] [PubMed]
- Gaudin V, Laurentie M. Application of total error approach to assess the performance of a biological method (ELISA) to detect nicarbazin residues in eggs. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877:2358-62. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)



