
Ovarian protection and safety of gonadotropin-releasing hormone agonist after cervical cancer surgery: systematic review and meta-analysis
Introduction
In recent years, there has been an increasing trend in the incidence of cervical cancer among younger women. Due to the general application of cervical cytology screenings among women at high risk for cervical human papilloma virus infection (1), many cervical malignant tumors are now diagnosed in the early stage of onset, which improves the prognosis of cervical cancer patients to a certain extent (2). The primary treatment for early cervical cancer is surgery, and in order to retain their fertility and improve their quality of life, some young patients choose to retain the ovary, and undergo a main PC (Paclitaxel + Cisplatin) chemotherapy regimen; however, the process of chemotherapy may cause serious damage to ovarian function (3).
A large number of studies (4,5) at home and abroad have shown that the application of the gonadotropin-releasing hormone agonist (GNRH-a) during chemotherapy has a protective effect on the ovarian function of cervical cancer patients who undergo PC (Paclitaxel + Cisplatin) chemotherapy regimen (6). Cervical cancer is a common clinical gynecological disease. The clinical symptoms of this disease include vaginal bleeding, and the disease mainly occurs in young and middle-aged women, the incidence of the disease is increasing year by year. In the clinical setting, the main methods for treating cervical cancer are surgery, radiotherapy, and chemotherapy, among which chemotherapy is an important treatment method. For patients who need supplementary chemotherapy after surgery, long periods of chemotherapy have a direct effect on ovarian secretion function, especially in young patients, which leads to the early aging of the ovaries, which seriously affects patients’ quality of life.
There is still controversy regarding the safety and efficacy of GNRH-a in the prevention of chemoradiotherapy-related ovarian function impairment. Studies (7,8) have shown that GNRH-a effectively protects ovarian function after chemotherapy in breast cancer patients. However, some studies (9,10) have suggested that GNRH-a does not provide ovarian protection. The reason may be related to the strong toxicity of the cyclophosphamide gonadads contained in the chemotherapy regimen, or it may be that GNRH-a causes incomplete pituitary-ovarian desensitization. The GNRH-a protects ovarian function by: (I) reducing the number of primary follicles entering all levels, putting the ovary in a pubertal state; (II) producing a low estrogen state, reducing ovarian perfusion, and thus reducing the damage caused by chemotherapy drugs; and (III) reducing apoptosis in ovarian cells by activating GnRH receptors or upregulating intragonadal anti-apoptotic molecules.
The bovine follicle stimulating hormone (bFSH), bovine estrogen 2 (bE2), inhibin B (INHB), anti-Mullerian hormone (AMH), and bovine antral follicle count (bAFC) are sensitive indicators used to assess ovarian function. The bE2 and bFSH are 2 early indicators, and are used in clinical settings to evaluate ovarian function. INHB is a direct indicator for predicting the ovarian reserve (INHB <40 pg/mL). Serum AMH levels indirectly reflect ovarian reserve function, and AMH is a reliable predictor of ovarian function. Compared to the other indicators, AMH can earlier and more accurately assess ovarian reserve function; Normal range of AMH is 0.7–6 g/L. The bAFC indirectly reflects the number of remaining follicles in the follicle pool and reflects the ovarian reserve function, and the bAFC (10).
In recent years, the incidence of cervical cancer shows a trend of younger, with the popularization of cervical cytology screening and people’s high-risk cervical human papillomavirus (HPV) infection is deepened, a lot of cervical malignant tumors in the early stage of the disease. Screening and diagnosis improve the prognosis of cervical cancer to a certain extent. Early cervical cancer is mainly treated with surgery. For some young patients, in order to preserve their fertility and improve their quality of life, some patients choose preservation. Radical ovarian cervical cancer surgery is supplemented with TP chemotherapy as the main program, but the function of preserved ovary may be seriously damaged in the process of chemotherapy, resulting in a serious decrease in the quality of life of patients. There are a lot of researches at home and abroad. The results showed that the application of GNRH-a in the course of chemotherapy can protect the ovarian function of patients with cervical cancer receiving TP chemotherapy. The effectiveness and safety of GNRH-a are not consistent, and there is great controversy. Therefore, it is very important to systematically evaluate the protection and safety of GNRH-a after cervical cancer surgery by using meta-analysis.
In this study, we analyzed the protective effect of the GNRH-a on ovarian protection after radical chemotherapy in women with cervical cancer aged 20–45 years old, and explored the value of the GNRH-a as an adjuvant drug used in 4–6 courses of TP chemotherapy to treat cervical cancer. We present the following article in accordance with the MOOSE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-928/rc).
Methods
Search strategy
PubMed, Cochrane Library, and Web of Science databases were searched to retrieve relevant articles. The following keywords: “cervical cancer”, “gonadotropin-releasing hormone agonist”, and “ovarian protection”, etc. Articles that had not been publicly published were not included in this study. Due to different national conditions and patient ethnic differences, only women aged 20–45 years old were analyzed, and other countries and human-specific controlled experiments were not included in this study (see Figure 1).
Inclusion criteria
To be eligible for inclusion in this meta-analysis, studies had to meet the following inclusion criteria: (I) comprise study subjects who were Chinese women aged 20–45 years, who had participated in a randomized controlled trial examining the use of radical cervical cancer chemotherapy and the GNRH-a; (II) include study subjects who had undergone chemotherapy with a TP regimen for 4 to 6 weeks, and 6 months after treatment; (III) we use a gold-standard pathological diagnosis; (IV) have been domestically published from January 2014 to January 2019 and include complete original data; and (V) have a large and representative sample size. The literature search and data retrieval were conducted independently by two assessors. If any disagreement arose, the assessors study reached a consensus through discussion; (VI) relevant research literature was included strictly according to PICOS standards. PICOS: P is the subject of study. The target group or representative of the subject is relevant to the subject; I is for interventions. Therapeutic interventions or observational measures used in the study population; C is for comparison group. Indicators representing control groups and treatment measures or observations; O indicates end. Representative achievement indicators and related issues; S is for research, and that is what is a study design, cohort study, case control or cross-sectional study. Experimental group: conventional treatment + GNRH-a; Control group: conventional treatment.
Exclusion criteria
Studies were excluded from this meta-analysis, if they met any of the following inclusion criteria: (I) the clinical data were incomplete or the judgment index of the outcome was not standard; (II) the diagnosis had not been made using the gold standard; (III) the article contained data that had been repeatedly published; (IV) the study did not include control group; (V) the study included studies with <20 patients; (VI) the subjects were also treated with radiotherapy.
Data extraction
The authors independent selection literature, through the discussion of inconsistent or submitted to the third party arbitration, extracted data including the clinical features of the subjects (the number of cases, sex ratio, average age, ovarian pathological type), intervention characteristics (intervention, hormone dosage, course of treatment), the results of the study (curative effect and adverse reaction, etc.).
Statistical analysis
The data entry and analysis were conducted for the meta-analysis using Stata (15.0) software. Heterogeneity was assessed by I2 values. The 95% confidence interval (CI) of the study subjects was calculated, and the RR (Relative Risk) values were used as the effect indexes, and the final results were analyzed. The heterogeneity between the studies was assessed using I2 statistics. Results of 25%, 50%, and 75% represented low, medium, and high heterogeneity, respectively. If I2<50% and P>0.1 between studies, the fixed-effects model was used, and if I2>50% and P<0.1, the chi-square analysis indicated study heterogeneity, and the random-effects models was used, and any possible heterogeneity was searched for by a subgroup analysis. In the sensitivity analysis, the included articles were removed 1 by 1 to determine whether the pooled effect values were stable and reliable. Funnel plots were made to assess publication bias in the included studies, and if large, it was further assessed using Begg’s plots and Egger’s test (see Figures 2,3).

Results
Retrieval results of literature search
Ultimately, 10 publicly published articles met the literature inclusion and exclusion criteria (11-20). The study subjects comprised 579 cervical cancer patients, aged 20–45 years, who all received 4–6 standardized courses of TP chemotherapy. Among the subjects, 253 started chemotherapy 10 to 15 d, 3.6 mg of the GNRH-a, once 4 courses at 28 d, while 42 patients also received a 21 d , and 4 people were not treated with the GNRH-a. All the patients received paclitaxel from 135–175 mg/m2, platinum cisplatin from 65–75 mg/m2, 160 cisplatin from 80–100 mg/m2, and 55 neida platin from 80–100 mg/m2. There were no significant differences in terms of bAFC, AMH, bE2, and bFSH before chemotherapy in all the included control and study groups, and the patients were followed-up for 6 months after chemotherapy. The basic information of the studies is summarized in Table 1. As shown in Figure 3, the included literatures were mainly distributed within the triangle range, and there was no obvious risk of literature publication bias (Figure 3).
Table 1
| Study | Study type | Case (N) | Age (years) | Tumor types | bFSH (IU/L) | bE2 (pg/mL) | AMH (μg/L) | bAFC (numbers) | Follow-up time (months) |
|---|---|---|---|---|---|---|---|---|---|
| Kado R 2020 | Review | 68 | 44.75±1.56 | Cervical cancer | 6.72±2.58 | 45.87±12.24 | 2.17±1.47 | 11.93±3.23 | 15.8 |
| Lee DY 2020 | Cohort | 45 | 38.51±3.56 | Cervical cancer | 6.73±5.51 | 44.97±11.93 | 2.19±1.51 | 12.05±3.25 | 15.4 |
| Lambertini M 2019 | Review | 90 | 51.72±2.26 | Cervical cancer | 6.74±2.68 | 45.32±12.46 | 2.19±1.52 | 11.73±3.55 | 10.4 |
| Chen H 2019 | Review | 67 | 47.12±1.25 | Cervical cancer | 6.72±2.58 | 45.87±12.24 | 2.17±1.47 | 11.93±3.23 | 18.2 |
| Cui W 2019 | Review | 55 | 42.18±4.22 | Cervical cancer | 6.12±1.55 | 58.75±17.03 | 2.24±1.55 | 10.55±2.25 | 20.1 |
| Zhong Y 2019 | RCT | 120 | 50.12±1.14 | Cervical cancer | 5.75±1.38 | 58.85±16.99 | 1.98±1.25 | 12.13±3.23 | 12.4 |
| Ma N 2020 | Animal research | 80 | 42.12±6.25 | Cervical cancer | 6.22±3.68 | 57.98±16.99 | 2.25±1.44 | 11.88±1.65 | 15.2 |
| Park CY 2014 | Cohort study | 70 | 47.33±2.56 | Cervical cancer | 6.02±1.88 | 58.75±17.03 | 2.21±1.15 | 11.65±3.34 | 7.5 |
| Scaruffi P 2019 | RCT | 75 | 48.19±3.21 | Cervical cancer | 5.35±2.43 | 49.88±10.24 | 2.38±1.02 | 12.33±3.45 | 11.2 |
| Akahori T 2019 | Review | 96 | 55.12±1.49 | Cervical cancer | 6.45±2.44 | 50.57±12.55 | 2.55±2.15 | 11.44±3.66 | 6.5 |
bFSH, bovine follicle stimulating hormone; bE2, bovine estrogen 2; AMH, anti-Mullerian hormone; bAFC, bovine antral follicle count; RCT, randomized control trial.
Comparison of bFSH levels between patients treated with GNRH-a and controls
In total, 8 studies compared bFSH levels between the GNRH-a group and controls group, and a statistically significant difference was found between the GNRH-a treatment group and the blank control group in terms of bFSH levels [odds ratio (OR) =1.82, 95% CI: 1.38–2.38; P<0.0001] (Figure 4).

Comparison of bE2 levels between patients treated with GNRH-a and controls
In total, 6 studies compared bE2 levels between GNRH-a group and controls group, and a statistically significant difference was found between the GNRH-a treatment group and the blank control group in terms of bE2 levels (OR =2.39, 95% CI: 1.69–3.37; P<0.00001) (Figure 5).
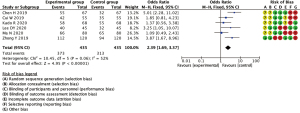
Comparison of AMH levels between patients treated with GNRH-a and controls
In total, 7 studies compared AMH levels between GNRH-a group and controls group, and a statistically significant difference was found between the GNRH-a treatment group and the blank control group in terms of AMH levels (OR =2.39, 95% CI: 1.71–3.34, P<0.00001) (Figure 6).

Comparison of bAFC levels between patients treated with GNRH-a and controls
In total 6 studies, compared bAFC levels between GNRH-a group and controls group, and a statistically significant difference was found between the GNRH-a treatment group and the blank control group in terms of bAFC levels (OR =2.11, 95% CI: 1.49–2.99; P<0.0001) (Figure 7).

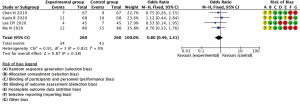
Incidence of coincidences
In total 6 studies, incidence of coincidences between GNRH-a group and controls group, and no statistically significant difference was found between the GNRH-a treatment group and the blank control group in terms of incidence of coincidences (OR =0.80, 95% CI: 0.49–1.31; P=0.38) (Figure 8).
Discussion
During the cytotoxic process of chemotherapy for cervical cancer, the cytotoxic effects of chemotherapeutic drugs can lead to the premature decline of ovarian function (21), reducing the number of follicles and leading to the fibrosis of ovarian tissue, and thus affecting the fertility of patients (22-25). The GNRH-a is an artificial GnRH derivative agent, which is mostly used in the treatment and recurrence prevention of endometriosis (26). It is a popular hormone drug in obstetrics and gynecology. The GNRH-a is available in a variety of dosage forms, and large dose of GNRH-a via subcutaneous injection can inhibit the maturation and recruitment of original follicles and reduce the toxicity of ovarian chemotherapeutic drug reactions, thus providing a protective effect to ovarian tissue (27-30).
We analyzed the ovarian protective effect of this drug in women with cervical cancer aged 20–45, and found significant differences in terms of bFSH, bE2, AMH and bAFC between the treatment and control groups (31). Further, we found that administering the GNRH-a during chemotherapy provided significant protective effects to the ovary tissue (32). All the subjects included in this study were Chinese women aged 20–45 years, who received a TP regimen and standardized chemotherapy; however, the types and measurements of the platinum drugs were quite different This study found that the difference of chemotherapy and platinum drugs and GNRH-a administration cycle were compared with bFSH, bE2, AMH and bAFC (33-35). Due to the actual measurements of individual chemotherapy and body surface areas, the included studies lacked clear quantitative data. Additionally, the follow-up time of 6 months was short. Thus, more studies need to be carried out (36).
This article had some limitations. First, the included studies were all retrospective controlled trials, and thus there is a greater probability of selection bias, which may have affected the meta-analysis. Second, most studies did not directly report the hazards ratio and its 95% CI, and the data extracted from the survival curve may not reflect the real data, which may have biased the merger results. Third, the operation level and operation mode of the operator were not completely consistent, which may have also affected the reliability of the results. Fourth, no comprehensive analysis of recent efficacy indicators, such as intraoperative bleeding volume, postoperative flow rate, hospitalization time or complications, was conducted (37-39).
At present, there is still controversy about the safety and effectiveness of the prevention of the GNRH-a caused by chemoradiotherapy (40). This study fully summarized and compared the existing studies, and the summary analysis of the GNRH-a under different administration cycles and TP platinum chemotherapy proves that the GNRH-a has some value in protecting ovarian function during chemotherapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-928/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-928/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Castillo JC, Haahr T, Martínez-Moya M, et al. Gonadotropin-releasing hormone agonist ovulation trigger-beyond OHSS prevention. Ups J Med Sci 2020;125:138-43. [Crossref] [PubMed]
- Castillo JC, Haahr T, Martínez-Moya M, et al. Gonadotropin-releasing hormone agonist for ovulation trigger - OHSS prevention and use of modified luteal phase support for fresh embryo transfer. Ups J Med Sci 2020;125:131-7. [Crossref] [PubMed]
- Driancourt MA, Briggs JR. Gonadotropin-Releasing Hormone (GnRH) Agonist Implants for Male Dog Fertility Suppression: A Review of Mode of Action, Efficacy, Safety, and Uses. Front Vet Sci 2020;7:483. [Crossref] [PubMed]
- Hershko Klement A, Navve D, Ghetler Y, et al. Gonadotropin releasing hormone agonist triggering for in vitro maturation cycles. Hum Fertil (Camb) 2020. [Epub ahead of print].
- Lin MH, Wu FS, Hwu YM, et al. Dual trigger with gonadotropin releasing hormone agonist and human chorionic gonadotropin significantly improves live birth rate for women with diminished ovarian reserve. Reprod Biol Endocrinol 2019;17:7. [Crossref] [PubMed]
- Kol S. A Rationale for Timing of Luteal Support Post Gonadotropin-Releasing Hormone Agonist Trigger. Gynecol Obstet Invest 2019;84:1-5. [Crossref] [PubMed]
- Rahmawati E, Yang WV, Lei YP, et al. Gonadotropin-releasing hormone agonist induces downregulation of tensin 1 in women with endometriosis. Acta Obstet Gynecol Scand 2019;98:222-31. [Crossref] [PubMed]
- Poggio F, Lambertini M, Bighin C, et al. Potential Mechanisms of Ovarian Protection with Gonadotropin-Releasing Hormone Agonist in Breast Cancer Patients: A Review. Clin Med Insights Reprod Health 2019;13:1179558119864584. [Crossref] [PubMed]
- Chen M, Luo L, Wang Q, et al. Impact of Gonadotropin-Releasing Hormone Agonist Pre-treatment on the Cumulative Live Birth Rate in Infertile Women With Adenomyosis Treated With IVF/ICSI: A Retrospective Cohort Study. Front Endocrinol (Lausanne) 2020;11:318. [Crossref] [PubMed]
- Bergenheim SJ, Perlman S, Banner-Voigt MLV, et al. Surgery, gonadotropin-releasing hormone agonist downregulation and in vitro fertilisation in women with infertility and endometriomas. Dan Med J 2020; [PubMed]
- Kado R, McCune WJ. Ovarian protection with gonadotropin-releasing hormone agonists during cyclophosphamide therapy in systemic lupus erythematosus. Best Pract Res Clin Obstet Gynaecol 2020;64:97-106. [Crossref] [PubMed]
- Lee DY, Kim JY, Yu J, et al. Prediction of Successful Ovarian Protection Using Gonadotropin-Releasing Hormone Agonists During Chemotherapy in Young Estrogen Receptor-Negative Breast Cancer Patients. Front Oncol 2020;10:863. [Crossref] [PubMed]
- Lambertini M, Horicks F, Del Mastro L, et al. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: From biological evidence to clinical application. Cancer Treat Rev 2019;72:65-77. [Crossref] [PubMed]
- Chen H, Xiao L, Li J, et al. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev 2019;3:CD008018. [PubMed]
- Cui W, Phillips KA. Conservative management of reproductive cancers. Ovarian protection during treatment. Best Pract Res Clin Obstet Gynaecol 2019;55:49-58. [Crossref] [PubMed]
- Zhong Y, Lin Y, Cheng X, et al. GnRHa for Ovarian Protection and the Association between AMH and Ovarian Function during Adjuvant Chemotherapy for Breast Cancer. J Cancer 2019;10:4278-85. [Crossref] [PubMed]
- Ma N, Chen G, Chen J, et al. Transient impact of paclitaxel on mouse fertility and protective effect of gonadotropin-releasing hormone agonist. Oncol Rep 2020;44:1917-28. [Crossref] [PubMed]
- Park CY, Jung SY, Lee KB, et al. The feasibility and efficacy of gonadotropin-releasing hormone agonists for prevention of chemotherapy induced ovarian failure in patient with gynecological malignancies. Obstet Gynecol Sci 2014;57:478-83. [Crossref] [PubMed]
- Scaruffi P, Stigliani S, Cardinali B, et al. Gonadotropin Releasing Hormone Agonists Have an Anti-apoptotic Effect on Cumulus Cells. Int J Mol Sci 2019;20:6045. [Crossref] [PubMed]
- Akahori T, Woods DC, Tilly JL. Female Fertility Preservation through Stem Cell-based Ovarian Tissue Reconstitution In Vitro and Ovarian Regeneration In Vivo. Clin Med Insights Reprod Health 2019;13:1179558119848007. [Crossref] [PubMed]
- Dong M, Sun L, Huang L, et al. Gonadotropin-releasing hormone agonist combined with hormone replacement therapy does not improve the reproductive outcomes of frozen-thawed embryo transfer cycle in elderly patients: a retrospective study. Reprod Biol Endocrinol 2020;18:73. [Crossref] [PubMed]
- Mohamad NV, Ima-Nirwana S, Chin KY. The effects of gonadotropin-releasing hormone agonist (buserelin) and orchidectomy on bone turnover markers and histomorphometry in rats. Aging Male 2020;23:327-34. [Crossref] [PubMed]
- Barbosa M, Paredes S, Machado MJ, et al. Pituitary apoplexy induced by gonadotropin-releasing hormone agonist administration: a rare complication of prostate cancer treatment. Endocrinol Diabetes Metab Case Rep 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Shim M, Bang WJ, Oh CY, et al. The Use of Gonadotropin-Releasing Hormone Agonist Does Not Affect the Development of Cardiovascular Disease in Prostate Cancer Patients: a Nationwide Population-Based Cohort Study. J Korean Med Sci 2020;35:e47. [Crossref] [PubMed]
- Davar R, Dashti S, Omidi M. Endometrial preparation using gonadotropin-releasing hormone agonist prior to frozen-thawed embryo transfer in women with repeated implantation failure: An RCT. Int J Reprod Biomed 2020;18:319-26. [Crossref] [PubMed]
- Tzoupis H, Nteli A, Androutsou ME, et al. Gonadotropin-Releasing Hormone and GnRH Receptor: Structure, Function and Drug Development. Curr Med Chem 2020;27:6136-58. [Crossref] [PubMed]
- Matsushima T, Akira S, Yoneyama K, et al. Recurrence of uterine adenomyosis after administration of gonadotropin-releasing hormone agonist and the efficacy of dienogest. Gynecol Endocrinol 2020;36:521-4. [Crossref] [PubMed]
- Atchia KS, Wallis CJD, Fleshner N, et al. Switching from a gonadotropin-releasing hormone (GnRH) agonist to a GnRH antagonist in prostate cancer patients: A systematic review and meta-analysis. Can Urol Assoc J 2020;14:36-41. [PubMed]
- Ghaffari F, Jahangiri N, Madani T, et al. Randomized controlled trial of gonadotropin-releasing hormone agonist microdose flare-up versus flare-up among poor responders undergoing intracytoplasmic sperm injection. Int J Gynaecol Obstet 2020;148:59-64. [Crossref] [PubMed]
- Qi Q, Luo J, Wang Y, et al. Effects of artificial cycles with and without gonadotropin-releasing hormone agonist pretreatment on frozen embryo transfer outcomes. J Int Med Res 2020;48:300060520918474. [Crossref] [PubMed]
- Aflatoonian A, Haghighi F, Hoseini M, et al. Does the repeat dose of gonadotropin-releasing hormone agonist trigger in polycystic ovarian syndrome improve in vitro fertilization cycles outcome? A clinical trial study. Int J Reprod Biomed 2020;18:485-90. [Crossref] [PubMed]
- Aghahoseini M, Alyasin A, Rashidi S, et al. The efficacy of gonadotropin-releasing hormone (GNRH) agonist before frozen embryo transfer in improving pregnancy outcome and decreasing miscarriage rate in hyperandrogenic polycystic ovary syndrome women: a randomized clinical trial. Minerva Ginecol 2020;72:212-8. [Crossref] [PubMed]
- Ali SS, Elsenosy E, Sayed GH, et al. Dual trigger using recombinant HCG and gonadotropin-releasing hormone agonist improve oocyte maturity and embryo grading for normal responders in GnRH antagonist cycles: Randomized controlled trial. J Gynecol Obstet Hum Reprod 2020;49:101728. [Crossref] [PubMed]
- Hernández-Jasso I, Domínguez-Del-Toro E, Delgado-García JM, et al. Recovery of sciatic nerve with complete transection in rats treated with leuprolide acetate: A gonadotropin-releasing hormone agonist. Neurosci Lett 2020;739:135439. [Crossref] [PubMed]
- Qu D, Li Y. Multiple-dose versus single-dose gonadotropin-releasing hormone agonist after first in vitro fertilization failure associated with luteal phase deficiency: A randomized controlled trial. J Int Med Res 2020;48:300060520926026. [Crossref] [PubMed]
- Ozaki R, Kumakiri J, Jinushi M, et al. Comparison of effect of preoperative dienogest and gonadotropin-releasing hormone agonist administration on laparoscopic cystectomy for ovarian endometriomas. Arch Gynecol Obstet 2020;302:969-76. [Crossref] [PubMed]
- Abufaraj M, Iwata T, Kimura S, et al. Differential Impact of Gonadotropin-releasing Hormone Antagonist Versus Agonist on Clinical Safety and Oncologic Outcomes on Patients with Metastatic Prostate Cancer: A Meta-analysis of Randomized Controlled Trials. Eur Urol 2021;79:44-53. [Crossref] [PubMed]
- Fontana F, Marzagalli M, Montagnani Marelli M, et al. Gonadotropin-Releasing Hormone Receptors in Prostate Cancer: Molecular Aspects and Biological Functions. Int J Mol Sci 2020;21:9511. [Crossref] [PubMed]
- Lim KI, Lee HS, Hwang JS. Changes in body mass index in boys with central precocious puberty over 2 years of gonadotropin-releasing hormone agonist therapy. Ann Pediatr Endocrinol Metab 2020;25:169-73. [Crossref] [PubMed]
- Cho AY, Ko SY, Lee JH, et al. Relationship between final adult height and birth weight after gonadotropin-releasing hormone agonist treatment in girls with central precocious puberty. Ann Pediatr Endocrinol Metab 2020;25:24-30. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)




