
Regulating mitochondrial homeostasis and inhibiting inflammatory responses through Celastrol
Introduction
Coronary heart disease has become the leading cause of death worldwide (1). Atherosclerosis, a chronic inflammation of the blood vessel wall caused by lipid accumulation, is an important pathological mechanism leading to the formation of coronary heart disease (2). Macrophage, as an innate immune cell, plays a central role in all stages of atherosclerosis development. In the early stages of the disease, monocytes in the blood migrate to tissues and become macrophages under the presence of oxidized low-density lipoprotein (3). Macrophages convert into foam cells by using their scavenger receptors to take up oxidized low-density lipoproteins (ox-LDLs). This process leads to the release of a large number of pro-inflammatory factors and chemokines, resulting in a severe inflammatory response (4). As the disease progresses, characteristic manifestations of vulnerable plaque, which are called fibrous cap, contain a large amount of collagen fibers, elastic fibers, and proteoglycans, with infiltration of macrophages and T cells. It is generally believed that apoptotic macrophages are cleared by exocytosis. However, with the accumulation of ox-LDL, fatty acids, and cholesterol, a large number of macrophages undergo apoptosis in atherosclerotic vulnerable plaques. These apoptotic macrophages are unable to be cleared in time due to severe inflammatory responses and impaired exocytosis. Therefore, they remain in the intima of the artery (5). A large number of apoptotic macrophages in vulnerable plaques cause a persistent and severe inflammatory response, which will eventually trigger plaque rupture or bleeding, leading to acute coronary syndrome (6). Essentially, the inflammatory response induced by macrophages plays a key role in the formation and exacerbation of atherosclerosis.
In recent years, compounds extracted from traditional Chinese herbal medicines have played an increasingly important role in the treatment of diseases. One of these compounds is Celastrol, a natural small molecule compound isolated from the root of the traditional Chinese herb “Thunder of God Vine” (Tripterygium wilfordii Hook F) (7). As early as 2007, Celastrol was listed with artemisinin, curcumin, capsaicin, and triptolide as 1 of the 5 most promising compounds to be developed into drugs in the 21st century. Recently, more and more studies have confirmed that Celastrol has a variety of pharmacological effects, including as an anti-oxidant, anti-cancer, and metabolism and immunity regulator (8-10). Based on the above pharmacological effects and a large amount of experimental data, Celastrol might be developed to treat autoimmune diseases, tumors, diabetes, and neurodegenerative diseases (9).
Mitochondria are double-membrane-bound subcellular organelles that produce energy currency, adenosine triphosphate (ATP), and are the main place for cells to perform aerobic respiration. Mitochondrial fission and fusion, which is also called mitochondrial quality control, is very critical for maintaining the normal morphology, function, and distribution of mitochondria. If an imbalance of the homeostasis between these processes occurs, it can lead to cell or organ dysfunction and abnormal mitochondrial redistribution (10). Mitochondrial fission is necessary to create new mitochondria, but it also helps quality control by removing damaged mitochondria and promoting apoptosis during cellular stress. This process is mediated by a member of the cytosolic dynamin family [dynamin-related protein 1 (Drp1) in mammals and dynamin 1 (Dnm1) in yeast]. Mitochondrial fusion helps reduce stress by promoting complementation between damaged mitochondria (11). Mitochondrial fusion proteins contain outer mitochondrial membrane (OMM) proteins and inner mitochondrial membrane (IMM) proteins. The outer membrane protein optic atrophy factor 1 (OPA1) participates in the fusion of the outer membrane of the mitochondria, while the inner membrane proteins mitofusin1 (Mfn1) and mitofusin2 (Mfn2) are involved in the fusion of the mitochondria’s inner membrane (12).
In this study, we found that Celastrol could significantly mitigate macrophage inflammation induced by the lipopolysaccharide (LPS) and reduce the release of pro-inflammatory cytokines and chemokines. In addition, we discovered for the first time that Celastrol could inhibit mitochondrial fission by promoting the phosphorylation of Drp1 at Ser637, and enhance mitochondrial fusion through the up-regulating of Mfn2 expression. At the same time, we also confirmed that Celastrol could up-regulate the expression of nuclear receptor Nur77, which may be related to its anti-inflammatory effect. Our current study reveals that Celastrol has an anti-inflammatory mechanism, which not only provides a theoretical basis for Celastrol to extenuate inflammation, but also provides a direction for the development of new natural compounds which reduce inflammation by regulating mitochondrial fission and fusion. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-7015/rc).
Methods
Materials
Our study was conducted using the following antibodies: rabbit polyclonal anti-iNOS, anti-GAPDH, anti-COX2, anti-Nur77, anti-LaminB1, anti-Drp1, anti-phospho-Drp1 (Ser637), and anti-Mfn2 purchased from Abcam (Cambridge, UK). We also used Rabbit polyclonal anti-p65, anti-phospho-p65, anti-ERK1/2, anti-phospho-ERK1/2, anti-JNK, anti-phospho-JNK, anti-p38, and anti-phospho-p38 purchased from Cell Signaling Technology Inc. (Danvers, MA, USA).
The chemical agents used in our study included Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin (Hyclone Laboratories Inc., Logan, UT, USA); Celastrol (MedChemExpress, Monmouth Junction, NJ, USA); LPS (Sigma-Aldrich, St. Louis, MI, USA); horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (H + L) and polyvinylidene fluoride (PVDF) (Beyotime Biotech, Jiangsu, China). The following kits were also used: BeyoECL Plus, Nuclear and Cytoplasmic Protein Extraction, Primary Antibody Dilution Buffer, and Mitochondrial Membrane Potential Assay with JC-1 (Beyotime Biotech, Jiangsu, China).
Cell culture and treatment
RAW264.7 cells (murine macrophage cell line) were purchased from iCell Bioscience Inc. (Shanghai, China). The RAW264.7 cells were propagated using DMEM, which contained 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin, and were maintained at 37 ℃ in a humidified 5% CO2 atmosphere. Cells were seeded at a density of 1×105 cells in a 6-well plate and were grown for 24 h before the experiment. RAW264.7 cells were pretreated with Celastrol for 40 min and then co-stimulated with 100 ng/mL of LPS and another 100 nmol/L of Celastrol for 24 h.
ELISA kit
Supernatants of cultured cells were collected after treatment and the secretion of cytokines and chemokines was measured using a commercial Enzyme-Linked Immunosorbent Assay (ELISA) kit (Boster Bio, Pleasanton, CA, USA) according to the manufacturer’s instructions.
RNA preparation and reverse transcription polymerase chain reaction (RT-PCR) test
Total RNA was isolated from the RAW264.7 cells through TRI-Solution (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Using 500 ng total RNA as raw material, complementary (cDNA) was synthesized by reverse transcription premix technology. A quantitative real-time PCR test was performed by using gene-specific primers (Table 1) and the PCR premix.
Table 1
| Name | Primer sequences |
|---|---|
| TNF-α | Forward: CTGAACTTCGGGGTGATCGG |
| Reverse: GGCTTGTCACTCGAATTTTGAGA | |
| IL-1β | Forward: GAAATGCCACCTTTTGACAGTG |
| Reverse: TGGATGCTCTCATCAGGACAG | |
| IL-6 | Forward: CTGCAAGAGACTTCCATCCAG |
| Reverse: AGTGGTATAGACAGGTCTGTTGG | |
| iNOS | Forward: GTTCTCAGCCCAACAATACAAGA |
| Reverse: GTGGACGGGTCGATGTCAC | |
| CCL-2 | Forward: TAAAAACCTGGATCGGAACCAAA |
| Reverse: GCATTAGCTTCAGATTTACGGGT | |
| CXCL-10 | Forward: CCAAGTGCTGCCGTCATTTTC |
| Reverse: GGCTCGCAGGGATGATTTCAA | |
| COX-2 | Forward: TGCACTATGGTTACAAAAGCTG |
| Reverse: TCAGGAAGCTCCTTATTTCC | |
| Nur77 | Forward: GAGTTCGGCAAGCCTACCAT |
| Reverse: GTGTACCCGTCCATGAAGGTG | |
| GAPDH | Forward: TGACCTCAACTACATGGTCTACA |
| Reverse: CTTCCCATTCTCGGCCTTG |
TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; iNOS, inducible nitric oxide synthase; CCL-2, chemokine (C-C motif) ligand 2; CXCL-10, chemokine (C-X-C motif) ligand 10; COX-2, cyclooxygenase 2; Nur77, nerve growth factor-induced gene B NGFI-B; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blot analysis
RAW264.7 cells were seeded in a 6-well plate in advance. After 24 h of drug treatment, the total protein was extracted from the cells with a phenylmethylsulphonyl fluoride (PMSF) lysis buffer (Roche, Basel, CH). After detecting the protein concentrations with a bicinchoninic acid (BCA) protein assay reagent (Thermo Fisher Scientific, Waltham, MA, USA), we separated proteins according to their different molecular weight with 8–12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred them onto a PVDF membrane.
After blocking with 5% skim milk, we put the PVDF membrane into an antibody incubation box which contained various primary antibodies diluted at the correct proportions and according to the relevant instructions. It was left at 4 ℃ overnight to allow the antigen and antibody to fully bind. The next day, the membrane was washed 3 times by using Tris-buffered saline with Tween-20 (TBST) for 5 minutes and then incubated with an HRP-linked secondary antibody before being diluted in TBST for 1 hour at room temperature. The protein was finally detected using a LAS-4000 mini system (Fujifilm, Tokyo, Japan).
Immunofluorescence
For the immunofluorescence of the RAW264.7 cells, they were seeded in 6-well plates with slides and underwent the following process: 500 μL of 4% paraformaldehyde added to each well (blocked for 15 min at room temperature), 0.3% Triton X-100 added (30 min at room temperature), PBS washing 5 min × 3 times, 5% antigen blocking goat serum added (volume fraction 0.02% Triton X-100 preparation) (30 min), JC-1 primary antibody incubation (volume fraction 0.02% Triton X-100 1:50 dilution) (1 h at room temperature), PBS washing 5 min × 3 times, FITC-labeled goat anti-rabbit IgG secondary antibody incubation (1:200 dilution) (1 h at room temperature and avoided light), and lastly they were mounted with VECTASHIELD mounting tablets containing 4',6-diamidino-2-phenylindole (DAPI). We then observed, photographed, and detected the expression of JC-1 under a fluorescence microscope. The primary antibody was then replaced with PBS or affinity-purified normal rabbit IgG as a negative control. The concentration of the negative control antibody was the same as that of the primary antibody, and the incubation conditions were also the same.
Statistical analysis
Our experiment was independently repeated 3 times to obtain the results which were expressed as mean ± standard deviation (SD). The values were evaluated by one-way analysis of variance, followed by a Duncan’s multiple range test using GraphPad Prism 4.0 software (GraphPad Software, Inc., San Diego, CA, USA). Differences of P<0.05 were considered significant.
Results
Construction of macrophage inflammation model
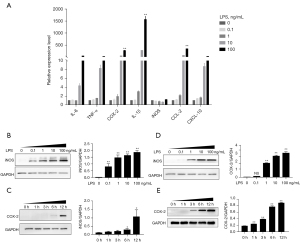
LPS was used to construct a macrophage inflammation model, and the inflammatory factors and chemotaxis were detected at different concentration (0, 0.1, 1, 10, 100 ng/mL) and time (0, 1, 3, 6, 12 h) points to factor the expression. Figure 1A shows that LPS can down-regulate the expression of IL-6, TNF-α, COX-2, IL-1β, iNOS, CCL-2, and CXCL-10 genes; Figure 1B,1C show that as the LPS concentration increases, the expression of the iNOS and COX-2 proteins increase; and Figure 1D,1E show that with an increase in the LPS administration time, the expression of the iNOS and COX-2 proteins increase.
Celastrol inhibits LPS-induced inflammatory response
The structure of Celastrol in Figure 2A. After the intervention of 100 nmol/L Celastrol (Figure 2B-2D), we found that inflammatory factors related to the cytokines IL-6, TNF-α, COX-2, IL-1β, and iNOS, as well as the chemokines CCL-2 and CXCL-10, were significantly down-regulated, suggesting that Celastrol could effectively reduce the inflammatory response of macrophages.

Study on the mechanism of Celastrol in regulating mitochondrial morphology and function
The mechanism of Celastrol in regulating mitochondrial morphology and function in Figure 3A. Celastrol promotes the phosphorylation modification of Drp1 at Ser637 (Figure 3B), inhibits mitochondrial division, reduces mitochondrial fragmentation, and exerts an anti-inflammatory effect. It can also promote mitochondrial fusion by up-regulating the expression of Mfn2 (Figure 3C) while at the same time adjusting the mitochondrial membrane potential (Figure 3D), thus improving the damaged mitochondrial function and playing a role in inhibiting inflammation.

Research on the mechanism of Celastrol regulating related inflammatory signaling pathways
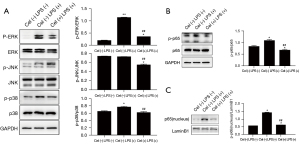
Our study found that Celastrol could inhibit inflammatory responses (Figure 4) by down-regulating both the phosphorylation level of extracellular signal-regulated kinase (ERK) and the p38 protein in the mitogen-activated protein kinase (MAPK) pathway, as well as the phosphorylation of the p65 protein in the nuclear factor-κB (NF-κB) pathway.
The effect of Celastrol on the expression of the nuclear receptor Nur77
In this study, different Celastrol concentrations and time points were set to analyze how it acts on macrophages. Nur77 mRNA and protein expression changes were detected using a qPCR test (Figure 5A,5B) and Western blot analysis (Figure 5C,5D). Figure 5 illustrates that Celastrol can not only induce the up-regulation of Nur77 mRNA and protein expression, but that its effect is dependent on concentrations and time points.

Discussion
Previous studies have shown that Celastrol has anti-inflammatory and anti-oxidative effects, inhibiting cell proliferation and inducing apoptosis. As atherosclerosis is a chronic inflammatory process, many mechanisms are involved in its occurrence and development, among which inflammatory mechanisms have been proven to be the main cause for triggering and promoting the disease (13,14). Macrophages are the main inflammatory cells in atherosclerotic plaque (13,14). Studies have shown that in atherosclerotic plaques of patients with acute coronary syndrome, especially in the “shoulder” of plaques, there are more macrophages and foam cells, with the number of macrophages having increased exponentially (15-17) when compared with that of stable plaques. Macrophages can produce free radicals and many tissue degrading proteases, such as elastase and matrix metalloproteinase, which can degrade collagen in the fiber cap, resulting in plaque rupture and thrombosis (16). The number of macrophages in the atherosclerotic plaque is positively correlated with the risk of plaque rupture. The accumulation of macrophages is a heavy marker and intervention target of unstable plaque (17). The results of our study show that Celastrol can effectively alleviate the inflammatory response of RAW264.7 induced by LPS.
Macrophages are the first line of defense against the invasion of exogenous pathogens. They can quickly identify pathogens and cause an immune inflammatory reaction to remove pathogens and maintain body homeostasis (18,19). With high plasticity and functional diversity, macrophages can polarize into cells with different phenotypes and functional states under different environmental stimuli. At present, they are widely recognized as classical activated macrophages (M1 macrophages) and alternative activated macrophages (M2 macrophages). LPS, as the main component of the cell wall of gram-negative bacteria, can be bound and recognized by the TLR4 protein. Previous studies (20-22) have shown that LPS stimulates macrophages to activate multiple inflammation-related signaling pathways, including MAPK, Janus kinase-signal transducer and activator of transcription (JAK-STAT), and the NF-κB. Some of the inflammatory factors these pathways produce include TNF-α, IL-6, iNOS, IL-1β, and so on. In human monocytes, stimulation of LPS activates NF-κB-p65, which then promotes the secretion of chemokines such as CCL-2, IL-8, and CCL-5. Studies (19-22) have shown that the activated protein kinase (AMPK) can pass NF-κB, while MAPK and JAK/STAT signaling pathways regulate inflammatory response. Swirski et al. (19) found that LPS, γ-interferon (INF)-γ, and microbial products such as granulocyte macrophage colony stimulating factors (GM-CSFs) can induce macrophages to polarize to a M1 phenotype, have stronger bactericidal and phagocytic power, and aggravate inflammatory reaction and tissue damage. Gordon et al. (20) also found that interleukin-4 (IL-4), TGF-β, and other immune-related complexes can stimulate macrophages to polarize to a M2 phenotype, down-regulate immune response, inhibit inflammatory response, and promote the repair of damaged tissue. Two polarizations run through the occurrence and development of as Dill et al. (21) found that in the early stage monocytes infiltrated into blood vessels and differentiated into M2 macrophages under the action of the macrophage colony stimulating factor (MCSF), promoting vascular remodeling and peripheral tissue repair. With the progress of inflammation, M1 macrophages gradually increase and dominate, inducing the production of the reactive oxygen species (ROS), promoting the secretion of NO, and accelerating TNF-α, IL-6, IL-1β, and other inflammatory factors. Woollard et al. (22) reported that Th2 cytokines can activate M2 macrophages, promote the formation of fibrous caps and improve the stability of plaques by driving the secretion of cytokines such as IL-4 and IL-13 in the plasma of advanced patients. Therefore, in order to further explore the anti-inflammatory activity and mechanism of Celastrol in the treatment of arteriosclerosis, this experiment used LPS to induce RAW264.7 cells to construct an in vitro inflammatory model, and observed the expression of M1/M2 macrophage-related inflammatory factors after Celastrol intervention by ELISA, real time PCR, and Western blot. It was found that Celastrol with different drug concentrations could significantly down-regulate the M1 macrophage pro-inflammatory factors IL-6 and TNF-α. The factors COX-2, IL-1 β, iNOS, CCL-2, and CXCL-10 also showed that Celastrol had good anti-inflammatory activity in vitro, which was confirmed by ELISA and real-time PCR.
Mitochondria constantly change their reticular structure through continuous fusion and division, which is called mitochondrial dynamics. This dynamic change not only has important physiological meaning for the exertion of its own function, but is considered to be the power center of both cardiomyocytes and mitochondria. This is because it is both a semi-autonomous organelle and an important signal organelle that participates in a variety of life-activity processes such as gene expression and cell apoptosis. However, the specific mechanism of mitochondrial dynamics on arteriosclerosis is not clear. Mitochondrial fusion proteins mainly include the dynein family GTPase proteins Mfn1 and Mfn2, which are the key proteins of mitochondrial outer membrane fusion (22,23). The optic atrophy associated protein 1 (Opa1) is a key fusion protein located in the mitochondrial membrane gap and mitochondrial intima, while the GTPase Drp1 and mitochondrial fission 1 protein (FIS1) are key proteins of mitochondrial division. Drp1 aggregates on the outer membrane of mitochondria and controls division through chemotaxis. The mechanism of FIS1 is to guide the transfer of Drp1 to the outer membrane of mitochondria and divide mitochondria into 2 new mitochondria through a series of actions. Recent studies (22-24) have shown that mitochondrial dynamics plays a key role in maintaining energy metabolism and that the dysfunction of its function can reduce the amount of ATP synthesized by mitochondria. Furthermore, the expression of the MTF2 gene can increase mitochondrial membrane potential, enhance the oxidation reaction of glucose, and increase respiratory chain products. Mfn1/Mfn2 are also closely related to apoptosis. Their specific mechanism may be to regulate the bid-mediated apoptosis pathway by regulating back on the outer membrane of mitochondria, inhibiting the expression of Drp1, and reducing the damage of caspase-9 (CASP9) activation to the mitochondria which is caused by ROS (and which may also be one of the causes of arteriosclerosis) (24). Celastrol can regulate the Drp1 and Mfn2 proteins, inhibit mitochondrial division, and promote fusion so as to reduce mitochondrial fragmentation and inhibit inflammation.
Mitochondria play an indispensable role in cell death, autophagy, immunity, and inflammation. Previous studies (25-28) have found that the orphan nuclear receptor Nur77 can induce apoptosis by targeting mitochondria. Because the characteristics of the vulnerable plaque of arteriosclerosis mainly include a large lipid core with thin fiber cap, an intense inflammatory response with increased apoptosis of vascular cells such as macrophages, etc., we believe that Nur77, which is highly up-regulated, may play an important regulatory role in promoting an inflammatory response and macrophage apoptosis. In recent years, Li et al. (25) found that under the induction of some apoptotic stimuli, Nur77 can combine with the retinoid X receptor (RXR) to form heterodimer, shift to the cytoplasm to target mitochondria, and then change the conformation of the Bcl-2 molecule from being an anti-apoptotic to pro-apoptotic molecule, thus promoting tumor cell apoptosis. Cheng et al. (26) also found that Nur77 can shift to mitochondria during myocardial ischemia-reperfusion injury, thus mediating cardiomyocyte apoptosis in the pathological process. At present, there are 2 opposing views on the effect of Nur77 on macrophage inflammatory responses in the pathological process of arteriosclerosis. Hanna et al. (27) believes that overexpressed Nur77 in macrophages can promote an inflammatory response (the researchers overexpressed Nur77 through lentivirus). However, according to the research results of Bonta et al. (28), Nur77 in macrophages can reduce lipid intake and the inflammatory reaction process. This study found that Celastrol can up-regulate the expression of Nur77 and be used as an Nur77 agonist, which provides a new direction for the study of Nur77 and the pathogenesis of arteriosclerosis.
In conclusion, Celastrol can up-regulate the expression of the Nur77 mRNA and protein, stimulate the expression of Nur77, and be used as a Nur77 agonist. At the same time, it can also significantly reduce the inflammatory response of macrophages, inhibit the release of related inflammatory factors and chemokines, regulate MAPK and NF-κB inflammatory signaling pathways, promote mitochondrial fusion, and change the level of mitochondrial membrane potential to regulate mitochondrial stability. All these factors confirm and clarify the anti-inflammatory role Celastrol can play in the potential treatment of arteriosclerosis.
Acknowledgments
Funding: This study was supported by Grants from the National Natural Science Foundation of China (81870338, 81570390).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-7015/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-7015/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-7015/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heller DJ, Coxson PG, Penko J, et al. Evaluating the Impact and Cost-Effectiveness of Statin Use Guidelines for Primary Prevention of Coronary Heart Disease and Stroke. Circulation 2017;136:1087-98. [Crossref] [PubMed]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95. [Crossref] [PubMed]
- Patel MJ, Blazing MA. Inflammation and atherosclerosis: disease modulating therapies. Curr Treat Options Cardiovasc Med 2013;15:681-95. [Crossref] [PubMed]
- Shao Q, Han F, Peng S, et al. Nur77 inhibits oxLDL induced apoptosis of macrophages via the p38 MAPK signaling pathway. Biochem Biophys Res Commun 2016;471:633-8. [Crossref] [PubMed]
- Kavurma MM, Rayner KJ, Karunakaran D. The walking dead: macrophage inflammation and death in atherosclerosis. Curr Opin Lipidol 2017;28:91-8. [Crossref] [PubMed]
- Shalhoub J, Falck-Hansen MA, Davies AH, et al. Innate immunity and monocyte-macrophage activation in atherosclerosis. J Inflamm (Lond) 2011;8:9. [Crossref] [PubMed]
- Chen X, Zhang B, Li J, et al. Celastrol attenuates incision-induced inflammation and pain associated with inhibition of the NF-κB signalling pathway via SARM. Life Sci 2018;205:136-44. [Crossref] [PubMed]
- Luo D, Guo Y, Cheng Y, et al. Natural product celastrol suppressed macrophage M1 polarization against inflammation in diet-induced obese mice via regulating Nrf2/HO-1, MAP kinase and NF-κB pathways. Aging (Albany NY) 2017;9:2069-82. [Crossref] [PubMed]
- Yang H, Chen D, Cui QC, et al. Celastrol, a triterpene extracted from the Chinese "Thunder of God Vine," is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res 2006;66:4758-65. [Crossref] [PubMed]
- Hu C, Huang Y, Li L. Drp1-Dependent Mitochondrial Fission Plays Critical Roles in Physiological and Pathological Progresses in Mammals. Int J Mol Sci 2017;18:144. [Crossref] [PubMed]
- Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science 2012;337:1062-5. [Crossref] [PubMed]
- Módis K, Bos E, Calzia EM, Van Goor H, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects. Br J Pharmacol 2014;171:2123-46. [Crossref] [PubMed]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95. [Crossref] [PubMed]
- Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol 2005;25:2255-64. [Crossref] [PubMed]
- Lutgens E, van Suylen RJ, Faber BC, et al. Atherosclerotic plaque rupture: local or systemic process? Arterioscler Thromb Vasc Biol 2003;23:2123-30. [Crossref] [PubMed]
- Boyle JJ. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol 2005;3:63-8. [Crossref] [PubMed]
- Broz P, Marsch S, Hunziker P. Targeting of vulnerable plaque macrophages with polymer-based nanostructures. Trends Cardiovasc Med 2007;17:190-6. [Crossref] [PubMed]
- Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol 2004;5:971-4. [Crossref] [PubMed]
- Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013;339:161-6. [Crossref] [PubMed]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010;32:593-604. [Crossref] [PubMed]
- Dill BD, Gierlinski M, Härtlova A, et al. Quantitative proteome analysis of temporally resolved phagosomes following uptake via key phagocytic receptors. Mol Cell Proteomics 2015;14:1334-49. [Crossref] [PubMed]
- Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010;7:77-86. [Crossref] [PubMed]
- Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta 2013;1833:1256-68. [Crossref] [PubMed]
- Shen T, Zheng M, Cao C, et al. Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem 2007;282:23354-61. [Crossref] [PubMed]
- Li Z, Li J, Zhu L, et al. Celastrol nanomicelles attenuate cytokine secretion in macrophages and inhibit macrophage-induced corneal neovascularization in rats. Int J Nanomedicine 2016;11:6135-48. [Crossref] [PubMed]
- Cheng Z, Völkers M, Din S, et al. Mitochondrial translocation of Nur77 mediates cardiomyocyte apoptosis. Eur Heart J 2011;32:2179-88. [Crossref] [PubMed]
- Hanna RN, Shaked I, Hubbeling HG, et al. NR4A1 (Nur77) Deletion Polarizes Macrophages Toward an Inflammatory Phenotype and Increases Atherosclerosis. Circ Res 2012;110:416-27. [Crossref] [PubMed]
- Bonta PI, Matlung HL, Vos M, et al. Nuclear receptor Nur77 inhibits vascular outward remodelling and reduces macrophage accumulation and matrix metalloproteinase levels. Cardiovasc Res 2010;87:561-8. [Crossref] [PubMed]
(English Language Editor: J. Goetz)


