
A case report of Maturity-onset diabetes of the young 12: large fragment deletion in ABCC8 gene with literature review
Introduction
Maturity-onset diabetes of the young (MODY) is a group of diabetes caused by gene defects related to insulin secretion. At present, 14 pathogenic genes have been found (1): HNF4A, GCK, HNF1A, PDX1, HNF1B, NEUROD1, KLF11, CEL, PAX4, INS, BLK, ABCC8, KCNJ11, APPL1. Most of patients lack specific clinical manifestations and require genetic testing to confirm the diagnosis. The MODY type diabetes is easily misdiagnosed as type 1 diabetes (T1DM) or type 2 diabetes (T2DM) in clinical practice. The classic diagnostic criteria included the following 3 criteria: family history of autosomal dominant diabetes, onset age of at least one person in the family <25 years old, and independent insulin treatment within 2 years after diagnosis of diabetes. However, about half of MODY patients confirmed by gene sequencing do not fully meet the traditional diagnostic criteria (2). So, it has been proposed that MODY patients should be screened among adolescents with autoimmune antibody negative onset of T1DM (3) and adolescents with type 2 diabetes (T2DM) lacking clinical features of metabolic disorders (4). In summary, there is still a lack of accepted screening criteria for MODY, and genetic testing remains the gold standard for diagnosing MODY (5).
The ATP-binding cassette transporter C8 gene (ABCC8) gene is located in chr11p with 39 exons encoding sulphonylurea receptor (SUR1) of KATP channel in the membrane of pancreatic β cells. Together with Kir6.2, SUR1 regulates the activity of KATP channel in the pancreatic β cell. Mutation of ABCC8 also leads to the deactivation or overactivation of the subunits of KATP channel. Deactivated SUR1 results in the shutdown of KATP channel and increased secretion of insulin (6), while activated SUR1 results in the opening of KATP channel and decreased secretion of insulin (7). Thus, mutation of ABCC8 could cause congenital hyperinsulinemic hypoglycemia as well as monogenetic diabetes. The onset age varies, including infants, MODY12, gestational diabetes, and T2DM (8).
MODY12 has a low incidence in the population, and with the widespread use of next-generation gene sequencing technology, cases of mody12 have been reported. However, the clinical characteristics of these patients are not completely consistent. They may have the onset of the disease in the young people, have a family history of diabetes, have poor treatment effect by insulin and are prone to hypoglycemia, and are effective for oral drug therapy such as sulfonylureas, metformin and sodium/glucose cotransporter 2 (SGLT2) inhibitors. In this paper, a patient with MODY12 caused by large fragment deletion of the ABCC8 gene was reported, and the clinical characteristics of MODY12 patients were analyzed in combination with the case, to help clinicians deepen their understanding of this disease. We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-807/rc).
Case presentation
A 22-year-old male, 175 cm in height, 44 kg in weight, body mass index (BMI) 14.4 kg/m2 was hospitalized for T1DM. He had been diagnosed with T1DM 4 years prior in the local hospital and received subcutaneous insulin infusion (Novolin R 10–12 U tid, glargine 16–20 U qn) with poor glucose control. He had been previously diagnosed with chronic viral hepatitis B, for which he received entecavir. His birth weight was 3.2 kg. His father had a history of diabetes for 30 years with insulin treatment. His diagnosis on admission was T1DM with chronic viral hepatitis B.
Treatment
On admission, the patient received subcutaneous insulin infusion (Novolin R 18 U qd before breakfast, 16 U qd before lunch + 16 U qd before supper, glargine 26 U qn) which yielded poor glucose control, thus he was converted to continuous insulin infusion (1.2 U/h, 16 U tid before meals). His blood glucose showed great variability with recurrent hypoglycemia and hyperglycemia.
Laboratory tests
Laboratory testing revealed the following: fasting blood glucose 23.41 mmol/L; abnormal liver function [alanine transaminase (ALT) 770 U/L, aspartate transaminase (AST) 360 U/L]; HBV-DNA quantitation 2.97×107 IU/mL; glycated hemoglobin [HbA1c] 12.9%; urine glucose (3+); urine ketone body (−); fasting C-peptide (FCP): 0.9 ng/mL (reference value range: 0.28–2.16 ng/mL); 1 h postprandial C-peptide (1-h CP): 0.8 ng/mL, 2 h postprandial C-peptide (2-h CP): 0.7 ng/mL; Glutamate decarboxylase antibody (GAD) (−); and islet cell antibody (ICA) (−).
Characteristics of the case
According to medical history and laboratory tests, characteristics of this case were summarized as follows: (I) adolescent onset; (II) weight loss; (III) paternal history of diabetes, no available health information about grandparents; (IV) poor glucose control under intensive insulin treatment, no ketoacidosis tendency; and (V) persistent FCP level, no postprandial secretion peak. Thus, MODY was suspected.
Genetic testing
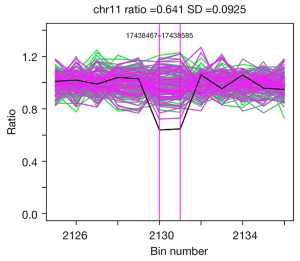
We extracted DNA from peripheral venous blood, then sequencing of exons of MODY [1–13]-related genes (HNF4α, GCK, HNF1α, PDX1, HNF1β, NEUROD1, KLF11, CEL, PAX4, INS, BLK, ABCC8, KCNJ11) were performed and compared with the reference sequences. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The results demonstrated a large heterozygous deletion at exon 17 of the ABCC8 gene in the chr11p15.1 region (Figure 1) which led to the disorder of downstream protein and resulted in the development of MODY 12.
Follow-up
Insulin therapy was gradually withdrawn after discharge and he was taking only glimepiride 2 mg qd since April 2018. His HbA1c was 6.1% and fasting plasma glucose was 7.49 mmol/L in July 2018. After stop drug treatment for 6 months, in February 2019, HbA1c was 5.9%, fasting plasma glucose was 6.53 mmol/L, 2 h postprandial plasma glucose 7.63 mmol/L, FCP 7.5 ng/mL, and 2 h postprandial C-peptide was 4.1 ng/mL. No treatment-related adverse events were observed during follow-up (Table 1).
Table 1
| Publication date | July, 2014 | February, 2018 | April, 2018 | July, 2018 | February, 2019 |
|---|---|---|---|---|---|
| Diagnosis | T1DM | Suspected MODY | Confirmed MODY12 | MODY12 | MODY12 |
| Treatment | Novolin R+Glargine (50 U/day) | Novolin R + Glargine (26 U/day) + Glimepiride (4 mg/day) | Glimepiride (2 mg/day) | Glimepiride (2 mg/day) | Drug withdraw for 6 months |
| C-peptide (ng/mL) | – | FCP:0.9; 1-h CP:0.8; 2-h CP:0.7 | – | – | FCP:7.5; 2-h CP:4.1; |
| Glycemic level | – | HbA1c: 12.9%; FBG: 23.41 mmol/L | – | HbA1c: 6.1%; FBG: 7.49 mmol/L | HbA1c: 5.9%; FBG: 6.53 mmol/L; 2hPG: 7.63 mmol/L |
| Hypoglycemia | – | Frequently | No | No | No |
T1DM, type 1 diabetes; MODY, Maturity-onset diabetes of the young; FCP, fasting C-peptide; 1-h CP, one hour postprandial C-peptide; 2-h CP, two-hour postprandial C-peptide; FBG, fasting blood glucose; 2hPG, two-hour postprandial glucose; HbA1c, glycosylated hemoglobin A1c.
Discussion
The MODY are a group of monogenetic diabetes characterized by the secretory dysfunction of pancreatic β cell, which consists of 14 phenotypes. And among these phenotypes, MODY 1–5 accounts for over 60%. In 2021, a study from China enrolled 76 Chinese families that met the clinical diagnostic criteria for MODY and found pathogenic mutations in 31 of them (40.79%). They were GCK (18.42%), HNF1α (15.79%), HNF4α (2.63%), KLF11 (1.32%), PAX4 and NEUROG3. This study suggests that the high incidence of the subtype in the Chinese population is consistent with that in the Caucasian population (9).
In this study, we reported a case of MODY 12 with a large heterozygous deletion at exon 17 of the ABCC8 gene. There is scarce evidence addressing ABCC8 mutation related adults’ cases in China, most of the previous reports have been congenital hyperinsulinemic hypoglycemia or neonatal diabetes mellitus (10). Among all the English literatures on PubMed database from 2010 to December 2021, 7 cases of MODY12 have been reported. As MODY12 is a familial hereditary disease, there are multiple patients in the family with different clinical manifestations. It is hard to list the clinical characteristics of all family members in one table. There were two Chinese families and one Russian patient similar to the one we reported. A 12-year-old male adolescent diabetic patient from China had similar diagnosis and treatment experience, who was first diagnosed with T1DM and finally diagnosed with MODY12 after genealogy analysis and gene sequencing correction. The patient started his disease with diabetic ketosis and received treatment with insulin for 4 months. His body weight increased by 8 kg and the total daily dosage of insulin was only 12 units. His blood glucose was well controlled and the c-peptide secretion level was significantly improved compared with the initial stage of the disease. After stopping insulin therapy and changing to Metformin hydrochloride (1.0 g/day) combined diet and exercise therapy, blood glucose was still well controlled (11). Another case report from China was a 15-year-old female patient with MODY12, the study revealed one novel missense variant of ABCC8 gene in Chinese families and indicated that the members of this family responded to treatment with sulfonylureas as previously seen in MODY (12). In 2016, a case report from Russia presented a case of MODY12 with an onset age of 27 years, in a non-obese man with epilepsy in anamnesis with normal C-peptide and recurrent hypoglycemia under low-dose insulin. After converting to modified release gliclazide combined with SGLT2 inhibitors, hypoglycemia was eliminated, and glycemic variability parameters were improved (13). These cases bear resemblance to the case in our study. Phenotypic characteristics of ABCC8-MODY: family history of autosomal dominant diabetes, early onset of diabetes (at least one member of the family with onset of diabetes <25 years old), no dependence on insulin treatment within 2 years of onset, negative autoimmune antibodies, impaired insulin secretion function but low insulin demand, frequent hypoglycemia and weight gain after insulin treatment. In our case, the proband’s father had a history of diabetes of over 30 years yet refused to undergo genetic testing, thus it is difficult to identify the origin of the gene mutation of the patients in our report.
Diabetes induced by ABCC8 mutation has a high heterogeneity of clinical manifestation. Kapoor et al. found that even family members who carried the same mutation site had different clinical manifestations including neonatal hypoglycemia, gestational diabetes, and adult onset diabetes mellitus with recurrent hypoglycemia (14). There are limited studies on the complications of MODY12 patients. A study of ABCC8-non neonatal diabetes mellitus (ABCC8-NNDM) patients collected data and found that about 50% of patients developed diabetic retinopathy, which may be related to SUR1 expression in the retina. Sulfonylureas can inhibit retinal neovascularization. The incidence of diabetic renal disease is low and is only seen in patients with three ABCC8 mutations. It should be noted that 34% of ABCC8-neonatal diabetes mellitus (ABCC8-NDM) patients were associated with neurological disease, due to high expression of KATP channel in both pancreas and nervous system, but the incidence of neurological complications was less than 1% in ABCC8-NNDM patients (15).
The ABCC8 gene mutation-related diabetes has low prevalence and various clinical manifestations. Its diagnosis requires expensive gene tests, thus could easily be misdiagnosed as T1DM or T2DM. Thus, it has been a challenge regarding how to screen out these patients. According to the case in our study and previous reports, we summarized the characteristics for which screening for ABCC8 is recommended: (I) onset of diabetes within 6 months of birth or congenital hyperinsulinemic hypoglycemia; (II) history of neonatal hypoglycemia or temporary neonatal diabetes mellitus; (III) those who meet the diagnostic criterion of MODY and (IV) low efficacy of insulin, recurrent hypoglycemia.
Sulphonylureas are the preferred medication for MODY12, and reports have also recommended nateglinide (16) and GLP-1 receptor agonists (17). Some cases also require insulin. Sulphonylureas shut down the KATP channel via SUR1 receptor in pancreatic β cells, reverse the opening of the KATP channel that causes ABCC8 mutation, and normalize the secretion of insulin. However, sulphonylureas do not work for all types of ABCC8-mutation related diabetes, a previous study found that 73.3% of the patients owing to ABCC8 variants with SUs got successful glucose control (15). The efficacy also depends on the structural and functional disorder caused by different mutations (18). Pooled evidence has suggested several characteristics of the therapeutic efficacy of sulfonylureas: (I) early initiation of sulfonylureas contributes to glucose control and prevents neonatal neuromuscular dysplasia and diabetic vascular complications (19); (II) the initial dosage should be calculated individually, which is 0.5–2.3 mg/kg/d as previously reported, some patients could have clinical remission even after withdrawing medication; glibenclamide, gliclazide, and glimepiride are all effective according to previous case reports. The patient in our study had already reached adulthood before changing their therapeutic regimen, and the efficacy of initial glimepiride (2 mg bid) was significantly better than insulin. Then, the dosage was gradually decreased to 2 mg and eventually withdrawn. Successful treatment was characterized by normal HbA1c, significantly reduced hypoglycemic events, and increased C-peptide secretion. Although other cases had been reportedly been previously treated successfully with sulfonylureas, the patient remained subject to the risk of hyperglycemia and was susceptible to relapse, especially when under stress from environment factors, unhealthy lifestyle, and other disease conditions.
At the end, we summarized some of “take away” information of MODY12. This was the first case of MODY12 induced by a large deletion of the ABCC8 gene. The patient was misdiagnosed with T1DM and had received insulin therapy for 4 years and prone to hypoglycemia or poor blood glucose control during insulin therapy. He attained clinical remission after converting to sulfonylureas. The limitation of our study was that we failed to complete a genetic study of the proband’s pedigree. The mechanism of abnormal insulin secretion caused by ABCC8 gene mutation and the clinical manifestations remain to be further studied. Exploring the pathological mechanism of congenital hyperinsulinemia converting to diabetes may be of great importance in revealing the pathogenesis of special types of diabetes as well as searching for novel therapeutic targets for diabetes.
Acknowledgments
Funding: The study was funded by Youth Project of Jiangxi Natural Science Foundation (No. 20202BABL2016018).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-807/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-807/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Urakami T. Maturity-onset diabetes of the young (MODY): current perspectives on diagnosis and treatment. Diabetes Metab Syndr Obes 2019;12:1047-56. [Crossref] [PubMed]
- Thanabalasingham G, Pal A, Selwood MP, et al. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care 2012;35:1206-12. [Crossref] [PubMed]
- Liu Y, Xie Z, Sun X, et al. A new screening strategy and whole-exome sequencing for the early diagnosis of maturity-onset diabetes of the young. Diabetes Metab Res Rev 2021;37:e3381. [Crossref] [PubMed]
- Kleinberger JW, Copeland KC, Gandica RG, et al. Monogenic diabetes in overweight and obese youth diagnosed with type 2 diabetes: the TODAY clinical trial. Genet Med 2018;20:583-90. [Crossref] [PubMed]
- Voevoda MI, Ivanova AA, Shakhtshneider EV, et al. Molecular genetics of maturity-onset diabetes of the young. Ter Arkh 2016;88:117-24. [Crossref] [PubMed]
- Koufakis T, Sertedaki A, Tatsi EB, et al. First Report of Diabetes Phenotype due to a Loss-of-Function ABCC8 Mutation Previously Known to Cause Congenital Hyperinsulinism. Case Rep Genet 2019;2019:3654618. [Crossref] [PubMed]
- Bowman P, Flanagan SE, Edghill EL, et al. Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia 2012;55:123-7. [Crossref] [PubMed]
- Riveline JP, Rousseau E, Reznik Y, et al. Clinical and metabolic features of adult-onset diabetes caused by ABCC8 mutations. Diabetes Care 2012;35:248-51. [Crossref] [PubMed]
- Liang H, Zhang Y, Li M, et al. Recognition of maturity-onset diabetes of the young in China. J Diabetes Investig 2021;12:501-9. [Crossref] [PubMed]
- Wang F, Han XY, Ren Q, et al. Effect of genetic variants in KCNJ11, ABCC8, PPARG and HNF4A loci on the susceptibility of type 2 diabetes in Chinese Han population. Chin Med J (Engl) 2009;122:2477-82. [PubMed]
- Lin L, Quan H, Chen K, et al. ABCC8-Related Maturity-Onset Diabetes of the Young (MODY12): A Report of a Chinese Family. Front Endocrinol (Lausanne) 2020;11:645. [Crossref] [PubMed]
- Tang C, Meng L, Zhang P, et al. Case Report: A Novel ABCC8 Variant in a Chinese Pedigree of Maturity-Onset Diabetes of the Young. Front Endocrinol (Lausanne) 2021;12:758723. [Crossref] [PubMed]
- Ovsyannikova AK, Rymar OD, Shakhtshneider EV, et al. ABCC8-Related Maturity-Onset Diabetes of the Young (MODY12): Clinical Features and Treatment Perspective. Diabetes Ther 2016;7:591-600. [Crossref] [PubMed]
- Kapoor RR, Flanagan SE, James CT, et al. Hyperinsulinaemic hypoglycaemia and diabetes mellitus due to dominant ABCC8/KCNJ11 mutations. Diabetologia 2011;54:2575-83. [Crossref] [PubMed]
- Li M, Han X, Ji L. Clinical and Genetic Characteristics of ABCC8 Nonneonatal Diabetes Mellitus: A Systematic Review. J Diabetes Res 2021;2021:9479268. [Crossref] [PubMed]
- Saito-Hakoda A, Yorifuji T, Kanno J, et al. Nateglinide is Effective for Diabetes Mellitus with Reactive Hypoglycemia in a Child with a Compound Heterozygous ABCC8 Mutation. Clin Pediatr Endocrinol 2012;21:45-52. [Crossref] [PubMed]
- Østoft SH, Bagger JI, Hansen T, et al. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care 2014;37:1797-805. [Crossref] [PubMed]
- Martin GM, Rex EA, Devaraneni P, et al. Pharmacological Correction of Trafficking Defects in ATP-sensitive Potassium Channels Caused by Sulfonylurea Receptor 1 Mutations. J Biol Chem 2016;291:21971-83. [Crossref] [PubMed]
- Marshall BA, Green RP, Wambach J, et al. Remission of severe neonatal diabetes with very early sulfonylurea treatment. Diabetes Care 2015;38:e38-9. [Crossref] [PubMed]


